

Amandla okuncipha anoma iyiphi iyunithi ubude ebusweni boketshezi abizwa ngokuthi i-surface tension, futhi iyunithi ingu-N.·m-1.

Isici sokunciphisa ukushuba kwesixazululi sibizwa ngokuthi umsebenzi ongaphezulu, futhi into enalesi sici ibizwa ngokuthi i-surface-active substance.
Into esebenza phezulu ekwazi ukubopha ama-molecule esixazululweni esinamanzi futhi yakhe ama-micelles nezinye izinhlangano, futhi ibe nomsebenzi ophezulu ongaphezulu, kuyilapho iphinde ibe nomthelela wokumanzisa, ukufaka ama-emulsifying, amagwebu, ukuwasha, njll. ibizwa ngokuthi i-surfactant.
I-Surfactant iyinhlanganisela ye-organic enesakhiwo esikhethekile kanye nempahla, engashintsha ngokuphawulekayo ukungezwani kobuso phakathi kwezigaba ezimbili noma ukungezwani okungaphezulu koketshezi (ngokuvamile amanzi), ngokumanzisa, ukukhihliza amagwebu, i-emulsifying, ukuwasha nezinye izakhiwo.
Ngokwesakhiwo, ama-surfactants anesici esivamile ngokuthi aqukethe amaqembu amabili emvelo ehlukene kuma-molecule awo.Ngakolunye uhlangothi kukhona uchungechunge olude lweqembu elingelona i-polar, elincibilika emafutheni futhi elingancibiliki emanzini, elaziwa nangokuthi iqembu le-hydrophobic noma iqembu elixosha amanzi.Iqembu elinjalo elixosha amanzi ngokuvamile lingamaketanga amade ama-hydrocarbon, ngezinye izikhathi futhi awe-organic fluorine, i-silicon, i-organophosphate, iketango le-organotin, njll. Ngakolunye uhlangothi iqembu elincibilikayo emanzini, iqembu le-hydrophilic noma iqembu elixosha uwoyela.Iqembu le-hydrophilic kumele libe ne-hydrophilic ngokwanele ukuqinisekisa ukuthi wonke ama-surfactants ancibilika emanzini futhi ane-solubility edingekayo.Njengoba ama-surfactants aqukethe amaqembu e-hydrophilic kanye ne-hydrophobic, angancibilika okungenani kwesinye sezigaba eziwuketshezi.Le mpahla ye-hydrophilic ne-lipophilic ye-surfactant ibizwa ngokuthi i-amphiphilicity.


I-Surfactant uhlobo lwama-amphiphilic molecule anawo womabili amaqembu e-hydrophobic kanye ne-hydrophilic.Amaqembu e-Hydrophobic of surfactants ngokuvamile akhiwa ama-hydrocarbon amaketanga amade, njenge-alkyl-chain eqondile C8~C20, i-alkyl-chain ye-branched C8~C20,alkylphenyl (inombolo ye-alkyl carbon tom ngu-8~16) nokunye okunjalo.Umehluko omncane phakathi kwamaqembu e-hydrophobic ikakhulukazi ekushintsheni kwesakhiwo samaketanga e-hydrocarbon.Futhi izinhlobo zamaqembu e-hydrophilic ziningi, ngakho-ke izakhiwo zama-surfactants zihlobene kakhulu namaqembu e-hydrophilic ngaphezu kobukhulu nokuma kwamaqembu e-hydrophobic.Izinguquko zesakhiwo samaqembu e-hydrophilic zinkulu kunalezo zamaqembu e-hydrophobic, ngakho-ke ukuhlukaniswa kwama-surfactants ngokuvamile kusekelwe esakhiweni samaqembu e-hydrophilic.Lokhu kuhlukaniswa kusekelwe ekutheni iqembu le-hydrophilic liyi-ionic noma cha, futhi lihlukaniswe laba i-anionic, i-cationic, i-nonionic, i-zwitterionic nezinye izinhlobo ezikhethekile zama-surfactants.

① I-Adsorption yama-surfactants ku-interfac
Ama-molecule e-surfactant angama-amphiphilic molecule anawo womabili amaqembu e-lipophilic nama-hydrophilic.Lapho i-surfactant incibilika emanzini, iqembu layo le-hydrophilic likhangwa emanzini futhi lincibilika emanzini, kuyilapho iqembu layo le-lipophilic lixoshwa ngamanzi futhi lishiya amanzi, okuholela ekukhanyisweni kwama-molecule e-surfactant (noma ama-ion) ekuxhumaneni kwezigaba ezimbili. , okunciphisa ukungezwani kobuso phakathi kwezigaba ezimbili.Uma ama-molecule e-surfactant (noma ama-ion) ekhangiswa kusixhumi esibonakalayo, kuyancipha kakhulu ukungezwani kobuso.
② Ezinye izici ze-adsorption membrane
Ingcindezi yangaphezulu ye-adsorption membrane: I-surfactant adsorption kusixhumi esibonakalayo segesi-uketshezi ukuze kwakhe ulwelwesi lwe-adsorption, njengokubeka ishidi elintantayo elingenakunyakaziswa kusixhumi esibonakalayo, ishidi elintantayo liphusha ulwelwesi lwe-adsorbent endaweni yesisombululo, futhi ulwelwesi lukhiqiza ingcindezi. eshidini elintantayo, elibizwa ngokuthi i-surface pressure.
I-Surface viscosity: Njengokucindezela kwendawo, i-surface viscosity yindawo eboniswa ulwelwesi lwamangqamuzana olungancibiliki.Imiswe indandatho yeplatinamu yensimbi enhle, ukuze indiza yayo ixhumane nobuso bamanzi ethangi, ijikeleze indandatho yeplatinamu, indandatho yeplatinamu yi-viscosity yesithiyo samanzi, i-amplitude ibola kancane kancane, ngokusho ukuthi i-viscosity yendawo ingaba kanjani. kukalwa.Indlela iwukuthi: okokuqala, ukuhlolwa kwenziwa emanzini ahlanzekile ukuze kulinganiswe ukubola kwe-amplitude, bese ukubola ngemva kokwakhiwa kolwelwesi olungaphezulu kukalwa, futhi i-viscosity yolwelwesi olungaphezulu itholakala umehluko phakathi kokubili. .
I-viscosity yangaphezulu ihlobene eduze nokuqina kolwelwesi olungaphezulu, futhi njengoba ulwelwesi lwe-adsorption lunomfutho ongaphezulu kanye ne-viscosity, kufanele lube nokunwebeka.Lapho umfutho ongaphezulu uphezulu futhi i-viscosity ye-adsorbed membrane iphezulu, ikhula imodulus yayo enwebekayo.I-elastic modulus ye-surface adsorption membrane ibalulekile enqubweni yokuqiniswa kwebhamuza.
③ Ukwakhiwa kwama-micelles
Izixazululo ezixubile zama-surfactants zithobela imithetho elandelwa yizixazululo ezifanele.Inani le-surfactant elikhangiswa ebusweni besisombululo liyakhula ngokugxilwa kwesisombululo, futhi lapho ukugxilisa ingqondo kufinyelela noma kwedlula inani elithile, inani le-adsorption alisakhushuki, futhi lawa ma-molecule angaphezu kwe-surfactant asesixazululo ngokungaqondile. ngendlela noma ngendlela ejwayelekile.Kokubili umkhuba kanye nethiyori kukhombisa ukuthi bakha izinhlangano ezixazululweni, futhi lezi zinhlangano zibizwa ngama-micelles.
I-Critical Micelle Concentration (CMC): I-Critical Micelle Concentration (CMC): I-concentration encane lapho ama-surfactants akha ama-micelles esixazululo ibizwa ngokuthi i-critical micelle concentration.
④ Amanani e-CMC ama-surfactants ajwayelekile.

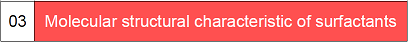
I-HLB isifinyezo sebhalansi ye-hydrophile lipophile, ekhombisa ibhalansi ye-hydrophilic ne-lipophilic yamaqembu e-hydrophilic kanye ne-lipophilic of the surfactant, okungukuthi, inani le-HLB lomuntu osebenza ngaphandle.Inani elikhulu le-HLB libonisa i-molecule ene-hydrophilicity eqinile kanye ne-lipophilicity ebuthakathaka;ngokuphambene, i-lipophilicity eqinile kanye ne-hydrophilicity ebuthakathaka.
① Ukunikezwa kwevelu ye-HLB
Inani le-HLB liyivelu ehlobene, ngakho-ke lapho inani le-HLB lithuthukiswa, njengezinga elijwayelekile, inani le-HLB le-wax kapharafini, elingenazo izici ze-hydrophilic, licaciswa ukuthi lingu-0, kuyilapho inani le-HLB le-sodium dodecyl sulfate, eliyi-hydrophilic. Okuncibilika kakhulu emanzini, kungu-40. Ngakho-ke, inani le-HLB lama-surfactants ngokuvamile lingaphakathi kwebanga elingu-1 ukuya ku-40. Ngokuvamile, ama-emulsifiers anamanani e-HLB angaphansi kuka-10 ane-lipophilic, kuyilapho lawo angaphezu kuka-10 e-hydrophilic.Ngakho-ke, ukuguqulwa kusuka ku-lipophilic kuya ku-hydrophilic cishe ku-10.
Ngokusekelwe kumanani e-HLB ama-surfactants, umqondo ojwayelekile wokusetshenziswa kwawo okungase kube khona ungatholwa, njengoba kuboniswe kuThebula 1-3.

Uketshezi olubili olungancibiliki, olulodwa luhlakazeka kwelinye njengezinhlayiya (amaconsi noma amakristalu awuketshezi) zakha uhlelo olubizwa ngokuthi i-emulsion.Lolu hlelo aluzinzile nge-thermodynamically ngenxa yokwanda kwendawo yomngcele weziphuzo ezimbili lapho i-emulsion ikhiwa.Ukuze wenze i-emulsion iqine, kuyadingeka ukwengeza ingxenye yesithathu - i-emulsifier ukunciphisa amandla ahlangene wesistimu.I-Emulsifier ingeye-surfactant, umsebenzi wayo oyinhloko ukudlala indima ye-emulsion.Isigaba se-emulsion esikhona njengamaconsi sibizwa ngokuthi isigaba esihlakazekile (noma isigaba sangaphakathi, isigaba esingapheli), kanti esinye isigaba esixhunywe ndawonye sibizwa ngokuthi i-dispersion medium (noma isigaba sangaphandle, isigaba esiqhubekayo).
① Ama-Emulsifiers nama-emulsions
Ama-emulsions avamile, isigaba esisodwa singamanzi noma isisombululo esinamanzi, esinye isigaba yizinto eziphilayo ezingaxubeki ngamanzi, njengamafutha, i-wax, njll. I-emulsion eyakhiwe ngamanzi namafutha ingahlukaniswa zibe izinhlobo ezimbili ngokusho kwesimo sabo sokuhlakazwa: amafutha. ihlakazekile emanzini ukuze yenze i-emulsion yohlobo lwamafutha emanzini, evezwe njenge-O/W (amafutha/amanzi): amanzi ahlakazekile emafutheni ukuze enze i-emulsion yohlobo lwamafutha emanzini, evezwe njenge-W/O (amanzi/amafutha).Uhlobo lwe-W/O/W oluyinkimbinkimbi lwamanzi-ngaphakathi-kawoyela emanzini kanye nohlobo lukawoyela emanzini-ngamafutha we-O/W/O ama-multi-emulsion amaningi nawo angase akheke.
Ama-Emulsifiers asetshenziselwa ukuzinzisa ama-emulsion ngokunciphisa ukungezwani kobuso futhi enze ulwelwesi olulodwa lwe-molecule yobuso.
Ku-emulsification yezidingo ze-emulsifier:
a: I-emulsifier kumele ikwazi ukukhangisa noma inothise ukuxhumana phakathi kwezigaba ezimbili, ukuze kunciphe ukungezwani phakathi kobuso;
b: I-emulsifier kufanele ikhokhise izinhlayiya, ukuze i-electrostatic repulsion phakathi kwezinhlayiya, noma yakhe ulwelwesi oluzinzile, oluvikelayo olubonakalayo ezizungeze izinhlayiya.
Ngakho-ke, into esetshenziswa njenge-emulsifier kufanele ibe namaqembu e-amphiphilic ukuze i-emulsify, futhi ama-surfactants angahlangabezana nale mfuneko.
② Izindlela zokulungiselela zama-emulsion kanye nezici ezithinta ukuzinza kwama-emulsion
Kunezindlela ezimbili zokulungisa ama-emulsion: enye iwukusebenzisa indlela yomshini ukuze uhlakaze uketshezi ezinhlayiyeni ezincane kolunye uketshezi, olusetshenziswa kakhulu embonini ukulungisa ama-emulsions;enye iwukuncibilikisa uketshezi esimweni samangqamuzana kolunye uketshezi, bese ulwenza luqoqeke kahle ukuze lwakhe ama-emulsions.
Ukuzinza kwe-emulsion yikhono lokulwa nokuhlanganiswa kwezinhlayiyana okuholela ekuhlukaneni kwesigaba.Ama-emulsions amasistimu angazinzi we-thermodynamically anamandla amakhulu amahhala.Ngakho-ke, lokho okubizwa ngokuthi ukuzinza kwe-emulsion empeleni yisikhathi esidingekayo ukuze uhlelo lufinyelele ukulingana, okungukuthi, isikhathi esidingekayo sokuhlukaniswa kolunye uketshezi ohlelweni ukuze kwenzeke.
Lapho ulwelwesi oluhlangene lobuso olunotshwala obunamafutha, ama-fatty acids nama-amine anamafutha kanye namanye ama-molecule e-polar organic, amandla olwelwesi aphakeme kakhulu.Lokhu kungenxa yokuthi, ongqimbeni oluhlangene lwe-adsorption lwama-emulsifier molecule nama-alcohol, ama-acids nama-amine namanye ama-molecule asezindaweni ezishisayo ukuze akhe "i-complex", ukuze amandla olwelwesi oluhlangene akhule.
Ama-Emulsifiers ahlanganisa ama-surfactants angaphezu kwamabili abizwa ngokuthi ama-emulsifiers axubile.I-emulsifier exubile ekhangisiwe endaweni esibonakalayo samanzi/samafutha;isenzo se-intermolecular singakha izinkimbinkimbi.Ngenxa yesenzo esiqinile se-intermolecular, ukungezwani kobuso kuncipha kakhulu, inani le-emulsifier elikhangiswa kusixhumi esibonakalayo liyanda kakhulu, ukwakheka kokuminyana kolwelwesi oluhlangene ubuso kuyanda, amandla ayanda.
Ukukhokhiswa ubuhlalu be-liquid kunomthelela omkhulu ekuzinzeni kwe-emulsion.Ama-emulsions azinzile, ubuhlalu bawo obuwuketshezi buvame ukushajwa.Uma kusetshenziswa i-emulsifier ye-ionic, i-ion emulsifier ekhangiswe kusixhumi esibonakalayo ineqembu layo le-lipophilic elifakwe esigabeni sikawoyela futhi iqembu le-hydrophilic lisesigabeni samanzi, ngaleyo ndlela lenze ubuhlalu obuwuketshezi bushajwe.Njengoba ubuhlalu be-emulsion obunecala elifanayo, bayaxoshana, akulula ukuhlanganisana, ukuze ukuqina kwande.Kungabonakala ukuthi ama-ion emulsifier adsorbed kakhulu ebuhlalu, ukushaja okukhulu, amandla amakhulu okuvimbela ubuhlalu kusuka ku-agglomeration, uhlelo lwe-emulsion luzinzile.
I-viscosity ye-emulsion dispersion medium inethonya elithile ekuzinzeni kwe-emulsion.Ngokuvamile, ukuphakama kwe-viscosity ye-dispersion medium, kuyanda ukuzinza kwe-emulsion.Lokhu kungenxa yokuthi i-viscosity ye-dispersion medium inkulu, enomthelela onamandla ekunyakazeni kwe-Brownian yobuhlalu obuwuketshezi futhi ibambezela ukungqubuzana phakathi kobuhlalu obuwuketshezi, ukuze uhlelo luhlale luzinzile.Imvamisa, izinto ze-polymer ezingancibilika kuma-emulsions zingakhuphula i-viscosity yesistimu futhi zenze ukuzinza kwama-emulsions kuphakame.Ngaphezu kwalokho, ama-polymers angakha futhi ulwelwesi oluqinile lwe-interfacial, okwenza uhlelo lwe-emulsion luzinze.
Kwezinye izimo, ukungezwa kwe-powder eqinile kungenza futhi i-emulsion ijwayele ukuzinza.Impushana eqinile isemanzini, emafutheni noma ku-interface, kuye ngamafutha, amanzi ngokumanzisa umthamo wempushana eqinile, uma impushana eqinile ingamanzi ngokuphelele ngamanzi, kodwa futhi imanzi ngamafutha, izohlala emanzini namafutha. esibonakalayo.
Impushana eqinile ayenzi i-emulsion izinze ngoba impushana eqoqwe kusixhumi esibonakalayo ithuthukisa ulwelwesi oluphakathi kobuso, olufana ne-adsorption ye-interfacial yama-molecule emulsifier, ngakho-ke lapho i-powder eqinile ihlelwa eduze kwesixhumi esibonakalayo, i-interface izinzile kakhulu. emulsion kuyinto.
Ama-surfactants anekhono lokukhulisa kakhulu ukuncibilika kwezinto eziphilayo ezingancibiliki noma ezincibilika kancane emanzini ngemva kokwenza ama-micelles esixazululweni esinamanzi, futhi isixazululo sisobala ngalesi sikhathi.Lo mphumela we-micelle ubizwa ngokuthi i-solubilization.I-surfactant engakhiqiza i-solubilization ibizwa ngokuthi i-solubilizer, kanti i-organic matter e-solubilized ibizwa ngokuthi i-solubilized matter.

I-Foam idlala indima ebalulekile ohlelweni lokugeza.Igwebu liwuhlelo lokuhlakazeka lapho igesi ihlakazwa khona oketshezini noma oluqinile, igesi ibe yisigaba esihlakazekile kanye noketshezi noma okuqinile njengendlela ehlakazayo, eyangaphambili ibizwa ngokuthi igwebu eliwuketshezi, kanti lena yakamuva ibizwa ngokuthi igwebu eliqinile. njengepulasitiki enegwebu, ingilazi enegwebu, usimende onegwebu njll.
(1) Ukwakheka kwegwebu
Ngegwebu lapha sisho iqoqo lamabhamuza omoya ahlukaniswe ulwelwesi oluwuketshezi.Lolu hlobo lwebhamuza luhlala lukhuphuka ngokushesha lufike endaweni ewuketshezi ngenxa yomehluko omkhulu wokuminyana phakathi kwesigaba esihlakazekile (igesi) kanye ne-dispersion medium (uketshezi), kuhlangene ne-viscosity ephansi yoketshezi.
Inqubo yokwenza ibhamuza iwukuletha inani elikhulu legesi oketshezini, futhi amabhamuza akuketshezi abuyele ngokushesha phezulu, akhe iqoqo lamabhamuza ahlukaniswe inani elincane legesi ewuketshezi.
Igwebu linezici ezimbili ezibalulekile mayelana ne-morphology: enye iwukuthi amabhamuza njengesigaba esihlakazekile ngokuvamile anesimo se-polyhedral, lokhu kungenxa yokuthi ezimpambanweni zamabhamuza, kunokuthambekela kokuthi ifilimu ewuketshezi ibe mncane ukuze amabhamuza abe mncane. i-polyhedral, lapho ifilimu ye-liquid iyancipha ngezinga elithile, iholela ekuqhumeni kwe-bubble;okwesibili ukuthi uketshezi oluhlanzekile alukwazi ukwakha igwebu elizinzile, uketshezi olungakha igwebu okungenani izingxenye ezimbili noma ngaphezulu.Izixazululo ezinamanzi zama-surfactants zijwayelekile kumasistimu athambekele ekukhiqizeni igwebu, futhi amandla azo okukhiqiza igwebu ahlobene nezinye izakhiwo.
Ama-surfactants anamandla acwebezelayo abizwa ngokuthi ama-foaming agents.Nakuba i-ejenti enegwebu inekhono elihle legwebu, kodwa i-foam eyakhiwe ingase ingakwazi ukugcina isikhathi eside, okungukuthi, ukuzinza kwayo akukuhle ngempela.Ukuze kugcinwe ukuzinza kwegwebu, ngokuvamile ku-ejenti enegwebu ukwengeza izinto ezingase zikhulise ukuzinza kwegwebu, into ibizwa ngokuthi i-foam stabilizer, i-stabilizer evame ukusetshenziswa yi-lauryl diethanolamine ne-dodecyl dimethylamine oxide.
(2) Ukuzinza kwegwebu
Igwebu liwuhlelo olungazinzile lwe-thermodynamically futhi inkambiso yokugcina ukuthi ingqikithi yendawo yoketshezi ngaphakathi kwesistimu iyancipha ngemva kokuphuka kwebhamuza namandla amahhala ehle.Inqubo yokukhipha amagwebu yinqubo lapho ulwelwesi oluwuketshezi oluhlukanisa igesi luba lugqinsi futhi lube mncane luze lunqamuke.Ngakho-ke, izinga lokuzinza kwe-foam linqunywa ngokuyinhloko ijubane lokukhishwa kwe-liquid namandla efilimu ye-liquid.Izici ezilandelayo nazo zinethonya kulokhu.
(3) Ukubhujiswa kwegwebu
Isimiso esiyisisekelo sokubhujiswa kwegwebu ukushintsha izimo ezikhiqiza igwebu noma ukuqeda izici zokuzinzisa zegwebu, ngakho-ke kukhona kokubili izindlela ezingokomzimba nezamakhemikhali zokuqeda amagwebu.
Ukukhipha amagwebu ngokomzimba kusho ukushintsha izimo zokukhiqizwa kwegwebu ngenkathi kugcinwa ukwakheka kwamakhemikhali esixazululo se-foam, njengokuphazamiseka kwangaphandle, izinguquko ekushiseni noma ekucindezelweni kanye nokwelashwa kwe-ultrasonic zonke izindlela ezisebenzayo zomzimba zokuqeda igwebu.
Indlela yokukhipha amagwebu ngamakhemikhali iwukwengeza izinto ezithile ukuze uxhumane ne-ejenti enegwebu ukuze kuncishiswe amandla efilimu ewuketshezi kugwebu futhi ngaleyo ndlela kuncishiswe ukuzinza kwegwebu ukuze kuzuzwe injongo yokukhipha amagwebu, izinto ezinjalo zibizwa ngokuthi ama-defoamers.Iningi lama-defoamers angama-surfactants.Ngakho-ke, ngokohlelo lokukhipha amagwebu, i-defoamer kufanele ibe nekhono eliqinile lokunciphisa ukushuba kwendawo, ukukhangiswa kalula phezulu, futhi ukusebenzisana phakathi kwama-athomu adsorption angaphezulu abuthakathaka, ama-adsorption molecule ahlelwe ngesakhiwo esixege kakhulu.
Kunezinhlobo ezahlukene ze-defoamer, kodwa ngokuyisisekelo, zonke zingama-non-ionic surfactants.Ama-surfactants angewona ama-ionic anezinto ezilwa nokugwaza eduze noma ngaphezulu kwendawo yawo yamafu futhi avame ukusetshenziswa njengama-defoamers.Utshwala, ikakhulukazi utshwala obunesakhiwo se-branching, ama-fatty acids nama-fatty acid esters, i-polyamides, i-phosphate esters, amafutha e-silicone, njll. nazo zivame ukusetshenziswa njengama-defoamers amahle kakhulu.
(4) Igwebu nokugeza
Akukho ukuxhumana okuqondile phakathi kwe-foam nokusebenza kokugeza futhi inani le-foam alibonisi ukuphumelela kokugeza.Isibonelo, ama-nonionic surfactants anezici ezimbalwa zokukhipha amagwebu kunezinsipho, kodwa ukususwa kwawo ukungcola kungcono kakhulu kunezinsipho.
Kwezinye izimo, i-foam ingaba usizo ekususeni ukungcola nokungcola.Isibonelo, lapho ugeza izitsha ekhaya, igwebu le-detergent lithatha amaconsi kawoyela futhi lapho kukhuhla umata, igwebu lisiza ukucosha uthuli, impushana nokunye ukungcola okuqinile.Ngaphezu kwalokho, igwebu ngezinye izikhathi lingasetshenziswa njengenkomba yokusebenza kwe-detergent.Ngenxa yokuthi amafutha anamafutha anomphumela ovimbelayo ku-foam ye-detergent, lapho kunamafutha amaningi kakhulu kanye ne-detergent encane kakhulu, akukho gwebu elizokhiqizwa noma i-foam yasekuqaleni izonyamalala.Igwebu futhi ngezinye izikhathi lingasetshenziswa njengenkomba yokuhlanzeka kwe-rinse, njengoba inani legwebu kusixazululo sokuhlanza livame ukuncipha ngokunciphisa okokuhlanza, ngakho-ke inani legwebu lingasetshenziswa ukuhlola izinga lokuwasha.
Ngomqondo obanzi, ukugeza kuyinqubo yokukhipha izingxenye ezingafuneki entweni ezowashwa futhi kuzuzwe injongo ethile.Ukugeza ngomqondo ojwayelekile kubhekisela enqubweni yokukhipha ukungcola ebusweni besithwali.Ekugezeni, ukusebenzisana phakathi kokungcola nesithwali kuba buthaka noma kuqedwe yisenzo sezinto ezithile zamakhemikhali (isb., okokuhlanza, njll.), ukuze inhlanganisela yokungcola nesithwali iguqulwe ibe yinhlanganisela yokungcola nesihlanzi, futhi ekugcineni ukungcola kuhlukanisiwe nomthwali.Njengoba izinto okufanele ziwashwe kanye nokungcola okufanele kukhishwe kuhlukahlukene, ukugeza kuyinqubo eyinkimbinkimbi kakhulu futhi inqubo eyisisekelo yokugeza ingavezwa ebuhlotsheni obulula obulandelayo.
I-Carrie··Dirt + Detergent= Isithwali + Ukungcola · Okokuhlanza
Inqubo yokugeza ngokuvamile ingahlukaniswa ngezigaba ezimbili: okokuqala, ngaphansi kwesenzo se-detergent, ukungcola kuhlukaniswa nomthwali wayo;okwesibili, ukungcola okuhlukanisiwe kuyahlakazwa futhi kumiswe phakathi nendawo.Inqubo yokuwasha iyinqubo ebuyiseleka emuva futhi ukungcola okuhlakazekile futhi okumisiwe phakathi nendawo kungase kuphinde kufakwe imvula kusukela ephakathi kuya entweni ewashwayo.Ngakho-ke, okokuhlanza okuhle kufanele kube nekhono lokuhlakaza nokumisa ukungcola futhi kuvimbele ukuhlelwa kabusha kokungcola, ngaphezu kwekhono lokususa ukungcola kumthwali.
(1) Izinhlobo zokungcola
Ngisho nangento efanayo, uhlobo, ukwakheka kanye nenani lokungcola kungahluka kuye ngokuthi imvelo isetshenziswa khona.Ukungcola komzimba kawoyela ikakhulukazi amafutha ezilwane nemifino kanye namafutha amaminerali (afana nowoyela ongahluziwe, uwoyela wokubasa, itiyela lamalahle, njll.), ukungcola okuqinile ikakhulukazi umlotha, umlotha, ukugqwala, i-carbon black, njll. Mayelana nokungcola kwezingubo, kukhona ukungcola emzimbeni womuntu, njengomjuluko, i-sebum, igazi, njll.;ukungcola okuvela ekudleni, njengamabala esithelo, amabala amafutha okupheka, amabala e-condiment, isitashi, njll.;ukungcola kwezimonyo, njenge-lipstick, i-nail polish, njll.;ukungcola okuvela emkhathini, njengothuli, uthuli, udaka, njll.;abanye, njengoyinki, itiye, ukunamathela, njll. Iza ngezinhlobo ezahlukene.
Izinhlobo ezihlukahlukene zokungcola ngokuvamile zingahlukaniswa zibe izigaba ezintathu eziyinhloko: ukungcola okuqinile, ukungcola okuwuketshezi kanye nokungcola okukhethekile.
① Ukungcola okuqinile
Ukungcola okuqinile okujwayelekile kufaka izinhlayiya zomlotha, udaka, umhlaba, ukugqwala kanye ne-carbon black.Iningi lalezi zinhlayiya zineshaji kagesi ebusweni bazo, iningi lazo lishajwe kabi futhi lingakhangiswa kalula ezintweni zefiber.Ukungcola okuqinile kuvamise ukuba nzima ukukuncibilika emanzini, kodwa kungahlakazwa futhi kumiswe ngezixazululo zokuhlanza.Ukungcola okuqinile okunamaphuzu amancane kunzima kakhulu ukukususa.
② Ukungcola okuwuketshezi
Ukungcola okuwuketshezi kuvame ukuncibilika kuwoyela, okuhlanganisa amafutha ezitshalo nawezilwane, ama-acid anamafutha, uphuzo oludakayo, uwoyela wamaminerali nama-oxide awo.Phakathi kwazo, amafutha ezitshalo nezilwane, ama-fatty acids kanye ne-alkali saponification angenzeka, kuyilapho ama-alcohols anamafutha, amafutha amaminerali angeyona i-saponified by alkali, kodwa angancibilika kuma-alcohol, ethers kanye ne-hydrocarbon organic solvents, kanye ne-detergent water solution emulsification and dispersion.Ukungcola okuwuketshezi okuncibilika uwoyela ngokuvamile kunamandla anamandla anezinto zefayibha, futhi kukhangiswa ngokuqinile emiculweni.
③ Ukungcola okukhethekile
Ukungcola okukhethekile kuhlanganisa amaprotheni, isitashi, igazi, uketshezi lomuntu njengokujuluka, i-sebum, umchamo nejusi yezithelo kanye nejusi yetiye.Iningi lalolu hlobo lokungcola lingakhangiswa ngamakhemikhali futhi ngokuqinile ezintweni zefiber.Ngakho-ke, kunzima ukugeza.
Izinhlobo ezahlukene zokungcola azivamile ukutholakala zodwa, kodwa zivame ukuxutshwa ndawonye futhi zikhangiswe entweni.Ukungcola ngezinye izikhathi kungase kufakwe i-oxidized, ukubola noma ukubola ngaphansi kwamathonya angaphandle, ngaleyo ndlela kudala ukungcola okusha.
(2) Ukunamathela kokungcola
Izingubo, izandla njll zingaba namabala ngoba kukhona uhlobo oluthile lokusebenzisana phakathi kwento nokungcola.Ukungcola kunamathela ezintweni ngezindlela ezihlukahlukene, kodwa akukho okungaphezu kokunamathela ngokomzimba namakhemikhali.
①Ukunamathela komle, uthuli, udaka, isihlabathi namalahle engutsheni ukunamathela ngokomzimba.Ngokuvamile, ngalokhu kunamathela kokungcola, futhi indima phakathi kwento enamabala ibuthakathaka, ukususwa kokungcola nakho kulula.Ngokusho kwamandla ahlukene, ukunamathela ngokomzimba kokungcola kungahlukaniswa ukunamathela komshini kanye nokunamathela kwe-electrostatic.
A: Ukunamathela komshini
Lolu hlobo lokunamathela ikakhulukazi lubhekisela ekunamatheleni kokungcola okuqinile (isib., uthuli, udaka nesihlabathi).Ukunamathela kwemishini kungenye yezindlela ezibuthakathaka zokunamathela kokungcola futhi kungasuswa cishe ngezindlela ezihlanzekile, kodwa lapho ukungcola kukuncane (<0.1um), kuba nzima kakhulu ukususa.
B: Ukunamathela kwe-electrostatic
Ukunamathela kwe-electrostatic kubonakala ikakhulukazi esenzweni sezinhlayiya zokungcola ezikhokhisiwe ezintweni ezishajwe ngokuphambene.Izinto eziningi ezine-fibrous zishajwa kabi emanzini futhi zinganamathela kalula kukho ukungcola okuthile okuchajiwe, njengezinhlobo ze-lime.Okunye ukungcola, nakuba kufakwe icala elibi, njengezinhlayiya zekhabhoni ezimnyama ezixazululweni ezinamanzi, zinganamathela kumafayibha ngamabhuloho e-ionic (ama-ion phakathi kwezinto eziningi eziphambene, ezisebenza kanye nazo ngendlela efana nebhuloho) ezakhiwe ama-ion aqondile emanzini (isb. , Ca2+, Mg2+ njll.).
Isenzo se-electrostatic sinamandla kunesenzo esilula somshini, okwenza ukususa ukungcola kube nzima uma kuqhathaniswa.
② Ukunamathela kwamakhemikhali
Ukunamathela kwamakhemikhali kubhekisela esimweni sokungcola okusebenza entweni ngamabhondi amakhemikhali noma e-hydrogen.Isibonelo, ukungcola okuqinile kwe-polar, amaprotheni, ukugqwala nokunye ukunamathela ezintweni ze-fiber, imicu equkethe i-carboxyl, i-hydroxyl, i-amide namanye amaqembu, la maqembu kanye nama-acids angcolile anamafutha, ama-alcohols anamafutha kulula ukwakha izibopho ze-hydrogen.Amandla amakhemikhali ngokuvamile anamandla futhi ukungcola ngakho-ke kuboshwe ngokuqinile entweni.Lolu hlobo lokungcola lunzima ukususa ngezindlela ezijwayelekile futhi ludinga izindlela ezikhethekile zokubhekana nalo.
Izinga lokunamathela kokungcola lihlobene nemvelo yokungcola ngokwayo kanye nemvelo yento enamathela kuyo.Ngokuvamile, izinhlayiya zinamathela kalula ezintweni ezine-fibrous.Okuncane ukuthungwa kokungcola okuqinile, kuqina ukunamathela.Ukungcola kwe-polar ezintweni ze-hydrophilic ezifana nekotini nengilazi kunamathela kakhulu kunokungcola okungeyona indawo.Ukungcola okungeyona indawo ezungezile kubambelela ngokuqinile kunokungcola kwe-polar, okufana namafutha asezindaweni ezishisayo, uthuli nobumba, futhi akulula ukukususa nokuhlanza.
(3) Indlela yokukhipha ukungcola
Inhloso yokugeza ukukhipha ukungcola.Esimweni esisezingeni lokushisa elithile (ikakhulukazi amanzi).Ukusebenzisa imiphumela ehlukahlukene yomzimba namakhemikhali we-detergent ukwenza buthaka noma ukuqeda umthelela wokungcola nezinto eziwashiwe, ngaphansi kwesenzo samandla athile emishini (njengokuhlikihla izandla, ukuphazamiseka komshini wokuwasha, umthelela wamanzi), ukuze ukungcola nezinto eziwashiwe. kusukela ngenhloso yokuqeda ukungcola.
① Indlela yokususa ukungcola okuwuketshezi
A: Ukumanzisa
Ukungcola okuwuketshezi ikakhulukazi kususelwa kuwoyela.Uwoyela ungcolisa izinto eziningi ezine-fibrous futhi usabalale kakhulu noma kancane njengefilimu kawoyela phezu kwempahla ene-fibrous.Isinyathelo sokuqala esenzweni sokugeza ukumanziswa kwendawo engaphezulu ngoketshezi lokuwasha.Ukuze senze umfanekiso, ubuso bomucu bungabhekwa njengendawo eqinile ebushelelezi.
B: Ibutho likawoyela - indlela yokugoqa
Isinyathelo sesibili esenzweni sokugeza ukukhishwa kwamafutha kanye namafutha, ukukhishwa kokungcola okuwuketshezi kufinyelelwa uhlobo oluthile lwekhoyili.Ukungcola okuwuketshezi ekuqaleni kwakukhona ebusweni ngendlela yefilimu kawoyela esakazwayo, futhi ngaphansi komphumela okhethekile wokumanzisa uketshezi oluwasha endaweni eqinile (okungukuthi, i-fiber surface), yagoqeka yaba ubuhlalu bamafutha igxathu negxathu. zathathelwa indawo uketshezi oluwashayo futhi ekugcineni zashiya indawo ngaphansi kwamandla athile angaphandle.
② Indlela yokususa ukungcola okuqinile
Ukususwa kokungcola okuwuketshezi ikakhulukazi ngokumanzisa okukhethekile komthwali wokungcola ngesisombululo sokuwasha, kuyilapho indlela yokususa ukungcola okuqinile ihlukile, lapho inqubo yokugeza ngokuyinhloko imayelana nokumanzisa kwenqwaba yokungcola kanye nengaphezulu layo elithwalayo ngokuwasha. isisombululo.Ngenxa yokukhanyiswa kwama-surfactants obhuqwini oluqinile kanye nendawo ethwalayo, ukusebenzisana phakathi kokungcola nendawo kuyancishiswa futhi amandla okunamathela obuningi bokungcola anciphe, ngaleyo ndlela ukungcola kususwe kalula ebusweni bendawo. umthwali.
Ngaphezu kwalokho, ukukhangisa kwama-surfactants, ikakhulukazi ama-ionic surfactants, ebusweni bokungcola okuqinile kanye nesithwali sawo sinamandla okwandisa amandla angaphezulu phezu kokungcola okuqinile kanye nesithwali sako, okulungele kakhulu ukususwa ukungcola.Izindawo eziqinile noma ngokuvamile ezine-fibrous ngokuvamile zishajwa ngendlela engafanele emithonjeni ye-aqueous ngakho-ke zingakha izendlalelo eziphindwe kabili ze-elekthronikhi ezindaweni ezingcolile noma ezindaweni eziqinile.Ngenxa yokunyanyiswa kwamacala a-homogeneous, ukunamathela kwezinhlayiya zokungcola emanzini endaweni eqinile kuyancipha.Uma i-anionic surfactant ingeziwe, ngoba ingakwazi ukukhulisa ngesikhathi esisodwa amandla angalungile ezinhlayiya zokungcola kanye nendawo eqinile, ukuxoshwa phakathi kwabo kuthuthukiswa kakhulu, amandla okunamathela kwezinhlayiyana ayancipha kakhulu, futhi ukungcola kulula ukususa. .
Ama-surfactants angewona ama-ionic akhangiswa ezindaweni eziqinile ezishajwa ngokuvamile futhi nakuba zingashintshi kakhulu amandla okusebenzelana kobuso, ama-adsorbed non-ionic surfactants avame ukwakha ukujiya okuthile kongqimba olukhangisiwe ngaphezulu okusiza ukuvimbela ukwakheka kabusha kokungcola.
Endabeni yama-cationic surfactants, ukukhangiswa kwawo kunciphisa noma kuqede amandla angaphezulu angemahle wenqwaba yokungcola kanye nendawo ethwalayo, okunciphisa ukunyanyeka phakathi kokungcola nongaphezulu futhi ngakho-ke akusizi ukususa ukungcola;ngaphezu kwalokho, ngemva kokukhangiswa endaweni eqinile, ama-cationic surfactants athambekele ekuguquleni indawo eqinile ibe yi-hydrophobic ngakho-ke awakukhuthazi ukumanzisa phezulu ngakho-ke ukuwashwa.
③ Ukususwa kwenhlabathi ekhethekile
Amaprotheni, isitashi, uketshezi lwabantu, ijusi yezithelo, ijusi yetiye nokunye ukungcola okunjalo kunzima ukususa ngama-surfactants avamile futhi kudinga ukwelashwa okukhethekile.
Amabala amaprotheni afana nokhilimu, amaqanda, igazi, ubisi kanye nendle yesikhumba kuvame ukujiya emicu nasekuwohlokeni futhi anamathele ngokuqinile.Ukungcola ngamaprotheni kungasuswa ngokusebenzisa ama-protease.I-enzyme protease idiliza amaprotheni othulini abe ama-amino acid noma ama-oligopeptides ancibilika emanzini.
Amabala esitashi aphuma ikakhulukazi ekudleni, okunye okunjengomhluzi, iglue njll. I-Amylase inomthelela omkhulu ku-hydrolysis yamabala esitashi, okwenza isitashi siphuke sibe ushukela.
I-Lipase igqugquzela ukubola kwe-triglycerides, okunzima ukuyikhipha ngezindlela ezivamile, njenge-sebum namafutha adliwayo, futhi iwaphule abe i-glycerol encibilikayo nama-fatty acids.
Amanye amabala anemibala evela kujusi wezithelo, amajusi wetiye, oyinki, i-lipstick njll. ngokuvamile kunzima ukuwahlanza kahle ngisho nangemva kokugeza okuphindaphindiwe.Lawa mabala angasuswa ukusabela kwe-redox nge-oxidizing noma i-ejenti yokunciphisa efana ne-bleach, ecekela phansi ukwakheka kwamaqembu akhiqiza umbala noma asiza ngombala futhi awehlise abe izingxenye ezincane ezincibilika emanzini.
(4) Indlela yokususa ibala yokuhlanza okomile
Okungenhla empeleni okwamanzi njengendlela yokugeza.Eqinisweni, ngenxa yezinhlobo ezahlukene zezingubo kanye nesakhiwo, ezinye izingubo zisebenzisa ukuwasha ngamanzi akulula noma akulula ukuzigeza zihlanzekile, ezinye izingubo ngemva kokugeza ngisho nokuguqulwa, ukufiphala, njll., isibonelo: imicu eminingi yemvelo imunca amanzi futhi kulula ukuvuvukala, futhi yomile futhi kulula ukushwabana, ngakho ngemva kokugeza izobe ikhubazekile;ngokugeza imikhiqizo yoboya futhi ngokuvamile avele shrinkage mkhuba, ezinye imikhiqizo yoboya nge ukugeza amanzi futhi kulula pilling, ukushintsha umbala;Abanye osilika abazizwa bezandla ziba kubi kakhulu ngemva kokugeza futhi balahlekelwe ukucwebezela kwabo.Kulezi zingubo ngokuvamile zisebenzisa indlela yokuhlanza okomile ukuze kukhishwe ukungcola.Okubizwa ngokuthi ukuhlanza okomile ngokuvamile kubhekisela endleleni yokugeza ku-solvents eziphilayo, ikakhulukazi ku-non-polar solvents.
Ukuhlanza okomile kuyindlela elula yokugeza kunokugeza ngamanzi.Ngenxa yokuthi ukuhlanza okomile akudingi isenzo esiningi semishini, akubangeli umonakalo, ukushwabana nokuguqulwa kwezingubo, kuyilapho ama-ejenti okuhlanza okomile, ngokungafani namanzi, awavamile ukukhiqiza ukunwetshwa nokunciphisa.Uma nje ubuchwepheshe busingathwa kahle, izingubo zingomiswa ngaphandle kokuhlanekezela, ukufiphala kombala kanye nokuphila kwesevisi okwandisiwe.
Mayelana nokuhlanza okomile, kunezinhlobo ezintathu ezibanzi zokungcola.
① Ukungcola okuncibilika ngamafutha Ukungcola okuncibilika ngamafutha kuhlanganisa zonke izinhlobo zikawoyela namafutha, okuwuketshezi noma okunamafutha futhi kungancibilika kuzihlanzisi ezomile zokuhlanza.
②Ukungcola okuncibilika emanzini Ukungcola okuncibilika emanzini kuyancibilika ezixazululweni ezinamanzi, kodwa hhayi ezintweni zokuhlanza ezomile, kukhangiswa engutsheni esesimweni esimanzi, amanzi ayahwamuka ngemva kokuna kwezulu eliqinile eliyimbudumbudu, njengosawoti we-inorganic, isitashi, amaprotheni, njll.
③Uwoyela namanzi ukungcola okungancibiliki Uwoyela nokungcola kwamanzi okungancibiliki akuncibiliki emanzini futhi akuncibiliki kuzincibilikisi zokuhlanza okomile, ezifana ne-carbon black, silicates ezinsimbi ezihlukahlukene nama-oxides, njll.
Ngenxa yemvelo ehlukene yezinhlobo ezahlukene zokungcola, kunezindlela ezihlukene zokususa ukungcola ohlelweni lokuhlanza okomile.Umhlabathi oncibilika ngamafutha, njengamafutha ezilwane nemifino, uwoyela wamaminerali namafutha, ancibilika kalula kuma-organic solvents futhi angasuswa kalula ekuhlanzeni okomile.Ukuncibilika okuhle kakhulu kwezinyibilikisi zokuhlanza okomile zamafutha namafutha empeleni kuvela kumandla e-van der Walls phakathi kwama-molecule.
Ukuze kukhishwe ukungcola okuncibilika emanzini okufana nosawoti we-inorganic, ushukela, amaprotheni kanye nomjuluko, inani elifanele lamanzi kufanele futhi lengezwe ku-ejenti yokuhlanza okomile, ngaphandle kwalokho ukungcola okuncibilika emanzini kunzima ukukususa ezingutsheni.Kodwa-ke, amanzi anzima ukuncibilika ku-ejenti yokuhlanza okomile, ngakho-ke ukwandisa inani lamanzi, udinga futhi ukwengeza ama-surfactants.Ukuba khona kwamanzi ku-ejenti yokuhlanza okomile kungenza ubuso bokungcola nezingubo zokugqoka bufakwe emanzini, ukuze kube lula ukuxhumana namaqembu e-polar of surfactants, okuhambisana nokukhanyiswa kwama-surfactants phezulu.Ngaphezu kwalokho, lapho ama-surfactants enza ama-micelles, ukungcola namanzi okuncibilika emanzini kungancibilika kuma-micelles.Ngaphezu kokwandisa okuqukethwe kwamanzi kwe-solvent yokuhlanza okomile, ama-surfactants angadlala indima ekuvimbeleni ukuphinda kufakwe ukungcola ukuze kuthuthukiswe umphumela wokukhipha ukungcola.
Ukuba khona kwamanzi amancane kuyadingeka ukuze kukhishwe ukungcola okuncibilika emanzini, kodwa amanzi amaningi angabangela ukuphazamiseka nokushwabana kwezinye izingubo, ngakho-ke inani lamanzi ku-ejenti yokuhlanza okomile kufanele libe ngokulinganisela.
Ukungcola okungancibiliki emanzini noma okungancibiliki uwoyela, izinhlayiya eziqinile njengomlotha, udaka, umhlaba kanye nesikhutha esimnyama, ngokuvamile kunamathiselwe engutsheni ngamandla e-electrostatic noma kuhlanganiswe namafutha.Ekuhlanzeni okomile, ukugeleza kwe-solvent, umthelela ungenza i-electrostatic force adsorption of dirt off, futhi i-ejenti yokuhlanza okomile ingancibilikisa uwoyela, ukuze inhlanganisela kawoyela nokungcola futhi inamathele engutsheni yezinhlayiya eziqinile zicime endaweni eyomile. -i-ejenti yokuhlanza, i-ejenti yokuhlanza eyomile emanzini amancane kanye nama-surfactants, ukuze lezo eziphuma ezinhlayiyeni zokungcola okuqinile zibe ukumiswa okuzinzile, ukuhlakazeka, ukuvimbela ukumiswa kabusha kwezingubo.
(5) Izinto ezithinta isenzo sokugeza
I-adsorption eqondisayo yama-surfactants kusixhumi esibonakalayo kanye nokunciphisa ukungezwani kwendawo (okuhlangene ubuso) yizici eziyinhloko ekukhishweni kokungcola okuwuketshezi noma okuqinile.Kodwa-ke, inqubo yokugeza iyinkimbinkimbi futhi umphumela wokugeza, ngisho nohlobo olufanayo lwe-detergent, luthonywa ezinye izici eziningi.Lezi zici zihlanganisa ukugxila kwe-detergent, izinga lokushisa, imvelo yenhlabathi, uhlobo lwe-fiber kanye nesakhiwo sendwangu.
① I-surfactant concentration
Ama-micelles ama-surfactants asesisombululo adlala indima ebalulekile enqubweni yokuwasha.Lapho ukugxila kufinyelela ku-critical micelle concentration (CMC), umphumela wokuwasha ukhuphuka kakhulu.Ngakho-ke, ukugxiliswa komshini wokuhlanza ku-solvent kufanele kube ngaphezu kwevelu ye-CMC ukuze kube nomthelela omuhle wokugeza.Kodwa-ke, lapho ukugxiliswa kwe-surfactant kungaphezulu kwevelu ye-CMC, ukwanda okukhuphukayo komphumela wokuwasha akubonakali futhi akudingekile ukukhulisa ukugxiliswa kwe-surfactant kakhulu.
Lapho ususa uwoyela ngokwenza i-solubilization, umphumela wokuxazululeka uyanda ngokugxila kwe-surfactant ekhulayo, ngisho nalapho ukugxilisa kungaphezu kwe-CMC.Ngalesi sikhathi, kuyatuseka ukusebenzisa okokuhlanza ngendlela yendawo endaweni eyodwa.Isibonelo, uma kukhona ukungcola okuningi kuma-cuffs kanye nekholomu yengubo, ungqimba lwe-detergent lungasetshenziswa ngesikhathi sokugeza ukuze kwandiswe umphumela we-solubilizing we-surfactant emafutheni.
②Izinga lokushisa linomthelela obaluleke kakhulu esenzweni sokuqeda ukungcola.Ngokuvamile, ukwandisa izinga lokushisa kusiza ukususwa kokungcola, kodwa ngezinye izikhathi izinga lokushisa eliphakeme kakhulu lingabangela ukungahambi kahle.
Ukwanda kwezinga lokushisa kusiza ukusakazeka kokungcola, amafutha aqinile afakwa kalula emulsified emazingeni okushisa ngaphezu kwendawo yokuncibilika kwayo futhi imicu iyanda ekuvuvukeni ngenxa yokwanda kwezinga lokushisa, konke okwenza kube lula ukususwa kokungcola.Kodwa-ke, ezindwangu ezihlangene, ama-microgaps phakathi kwemicu ayancishiswa njengoba imicu ikhula, okulimaza ukukhishwa kokungcola.
Ukushintsha kwezinga lokushisa kuphinde kuthinte ukunyibilika, inani le-CMC kanye nosayizi we-micelle wama-surfactants, ngaleyo ndlela kuthinte umphumela wokuwasha.I-solubility yama-surfactants anamaketanga ekhabhoni amade iphansi emazingeni okushisa aphansi futhi ngezinye izikhathi ukuncibilika kuphansi ngisho kunevelu ye-CMC, ngakho-ke izinga lokushisa lokugeza kufanele liphakanyiswe ngendlela efanele.Umthelela wezinga lokushisa kunani le-CMC kanye nosayizi we-micelle uhlukile kuma-ionic nama-non-ionic surfactants.Kuma-surfactants e-ionic, ukwanda kwezinga lokushisa ngokuvamile kwandisa inani le-CMC futhi kunciphisa usayizi we-micelle, okusho ukuthi ukugxila kwe-surfactant kusixazululo sokuwasha kufanele kunyuswe.Kuma-surfactants angewona ama-ionic, ukunyuka kwezinga lokushisa kuholela ekwehleni kwevelu ye-CMC kanye nokwenyuka okuphawulekayo kwevolumu ye-micelle, ngakho-ke kusobala ukuthi ukunyuka okufanelekile kwezinga lokushisa kuzosiza i-surfactant engeyona i-ionic ukuthi isebenzise umphumela wayo osebenza phezulu. .Nokho, izinga lokushisa akufanele lidlule iphuzu layo lefu.
Ngamafuphi, izinga lokushisa eliphezulu lokuwasha lincike ekwakhiweni komshini wokuwasha kanye nento ewashwayo.Ezinye izinto zokuhlanza zinomphumela omuhle wokuhlanza ekamelweni lokushisa, kanti ezinye zinezihlanzi ezihluke kakhulu phakathi kokugeza okubandayo nokushisayo.
③ Igwebu
Kuyisiko ukudida amandla anegwebu nomphumela wokuwasha, ukholelwa ukuthi okokuhlanza okunamandla acwebezelayo kunomphumela omuhle wokugeza.Ucwaningo luye lwabonisa ukuthi abukho ubuhlobo obuqondile phakathi komphumela wokuwasha kanye nenani legwebu.Isibonelo, ukugeza ngezihlanzi ezinegwebu eliphansi akuphumelelanga kangako kunokugeza ngezihlanzi ezinegwebu eliphezulu.
Nakuba i-foam ingahlobene ngokuqondile nokugeza, kunezikhathi lapho kusiza ukususa ukungcola, isibonelo, lapho ugeza izitsha ngesandla.Lapho kukhuhla okhaphethi, igwebu lingase futhi lisuse uthuli nezinye izinhlayiya zokungcola okuqinile, ukungcola kukakhaphethi kubala ingxenye enkulu yothuli, ngakho-ke ama-agent okuhlanza ukhaphethi kufanele abe nekhono elithile lokugwebu.
Amandla akhihliza amagwebu nawo abalulekile kuma-shampoos, lapho igwebu elihle elikhiqizwa uketshezi ngesikhathi se-shampoo noma ukugeza lishiya izinwele zizizwe zigcotshiwe futhi zikhululekile.
④ Izinhlobonhlobo zemicu kanye nezakhiwo ezibonakalayo zezindwangu
Ngaphandle kwesakhiwo samakhemikhali semicu, ethinta ukunamathela nokususwa kokungcola, ukubonakala kwezintambo kanye nenhlangano yentambo nendwangu kunomthelela ekukhipheni kalula ukungcola.
Izikali zemicu yoboya kanye namaribhoni ayisicaba agobile emicu kakotini kungenzeka ukuthi aqongelele ukungcola kunemicu ebushelelezi.Isibonelo, i-carbon black stained kumafilimu e-cellulose (amafilimu e-viscose) kulula ukuwasusa, kuyilapho i-carbon black stained ezindwangu zikakotini kunzima ukuyigeza.Esinye isibonelo ukuthi izindwangu ezimfishane ezenziwe nge-polyester zivame ukuqongelela amabala kawoyela kunezindwangu ze-fiber ende, futhi amabala kawoyela ezindwangu ezimfishane nawo anzima kakhulu ukuwasusa kunamabala kawoyela ezindwangu ezinde.
Izintambo eziphothiwe ngokuqinile nezindwangu eziqinile, ngenxa yegebe elincane phakathi kwemicu, zingamelana nokuhlasela kokungcola, kodwa okufanayo kungavimbela uketshezi oluwasha ukuba lungabandakanyi ukungcola kwangaphakathi, ngakho-ke izindwangu eziqinile ziqala ukumelana nokungcola okuhle, kodwa uma sezingcolile. ukuwasha nakho kunzima kakhulu.
⑤ Ukuqina kwamanzi
Ukugcwala kwe-Ca2+, Mg2+ namanye ama-ion ensimbi emanzini kunethonya elikhulu emthelela wokuwasha, ikakhulukazi lapho ama-anionic surfactants ehlangana ne-Ca2+ kanye ne-Mg2+ ions akha usawoti we-calcium ne-magnesium ongancibiliki kalula futhi uzonciphisa ukubola kwawo.Emanzini aqinile, noma ngabe ukugcwala kwe-surfactant kuphezulu, i-detergency iseyimbi kakhulu kune-distillation.Ukuze lowo osebenza emanzini abe nomthelela omuhle kakhulu wokuwasha, ukugcwala kwama-Ca2+ ion emanzini kufanele kwehliselwe ku-1 x 10-6 mol/L (CaCO3 kuya ku-0.1 mg/L) noma ngaphansi.Lokhu kudinga ukungezwa kwezithambisa ezinhlobonhlobo ku-detergent.
Isikhathi sokuthumela: Feb-25-2022



