Uhlu Lokuphakathi kwalesi sihloko:
1. Ukuthuthukiswa kwama-amino acid
2. Izakhiwo ezakhiwo
3. Ukwakheka kwamakhemikhali
4.Classification
5. Synthesis
I-6. Izakhiwo ze-Physicochemical
7. Ubuthi
8. Umsebenzi wokulwa namagciwane
9. Izakhiwo ze-Rheological
10. Izicelo embonini yezimonyo
11. Izicelo ezimonweni zansuku zonke
I-Amino acid Surfactants (AAS)Ingabe isigaba se-surfactants esakhiwa ngokuhlanganisa amaqembu e-hydrophobic nge-amino acid eyodwa noma ngaphezulu. Kulokhu, ama-amino acid angaba sonnike noma athathwe kumaprotheni hydrolysterates noma imithombo efanayo evuselelekayo. Leli phepha lihlanganisa imininingwane yezindlela eziningi zokwenziwa ze-AAS kanye nomphumela wemizila ehlukene kwizakhiwo ze-physichicochemical zemikhiqizo yokuphela, kufaka phakathi i-solubity, ukuqina kokusakaza, ubuthi kanye ne-biodergadability. Njengesigaba se-surficacters ekwandiseni ukufunwa, ukuguquguquka kwamanye amazwe ngenxa yesakhiwo sazo esiguquguqukayo kunikeza inani elikhulu lamathuba okuhweba.
Njengoba kunikezwe ukuthi ama-surfactants asetshenziswa kabanzi kuma-detegents, ama-emulsifiers, ama-inhibitorion, ukuvuselelwa kwamafutha aphezulu kanye nemithi yokubuyiselwa kwemithi, abacwaningi abakaze bayeke ukunaka ama-surfactants.
Ama-surfactants yimikhiqizo emele kakhulu yamakhemikhali edliwa ngamanani amakhulu nsuku zonke emhlabeni wonke futhi abe nomthelela omubi endaweni yasemanzini.Ucwaningo lukhombisile ukuthi ukusetshenziswa okusakazeke kakhulu kwama-surfactants wendabuko kungaba nomthelela omubi emvelweni.
Namuhla, okungeyona ubuthi, i-biodegracatity kanye nokuqina kwe-biocomtic cishe kubaluleke kakhulu kubathengi njengombuso kanye nokusebenza kwama-surfility.
I-Biosurfactants yilezi zinto ezenziwa ngemvelo ezizimele ezizimele ezisetshenziswa ngokwemvelo ezihlanganiswe ama-microorganisms anjengegciwane, isikhunta, nemvubelo, noma i-extracellulayly.Ngakho-ke, ama-biosurfactants angalungiswa futhi yi-molecular design ukuze alungise izinhlaka zemvelo ze-amphiphilic, ezinjenge-phospholipids, i-alkyl glycosides kanye nama-acyl amino acid.
Ama-Amino acid Surfactants (AAS)kungenye yezinto ezisetshenziswayo ezijwayelekile, ezivame ukukhiqizwa kusuka ezintweni ezingavuthiwe zezilwane noma zezomnotho. Eminyakeni engamashumi amabili edlule, ama-AAS adonsele inzalo enkulu kososayensi njengoba ama-varen surfactants, hhayi kuphela ngoba angenziwa kuphela ngoba angenawo amandla ancipha kalula futhi abe nokonakala ngemikhiqizo, abenze baphephe imvelo.
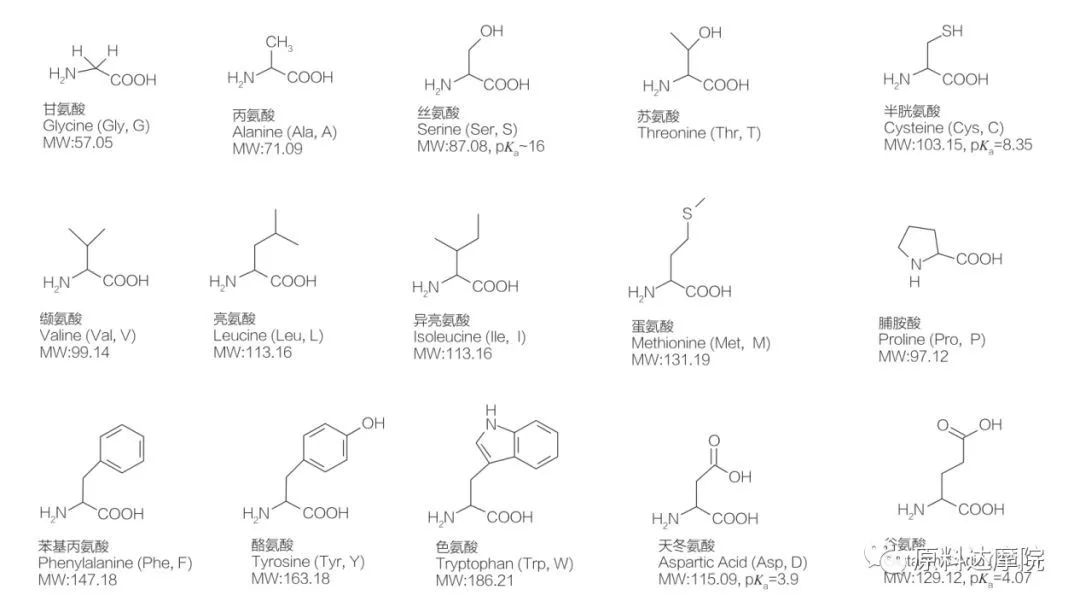
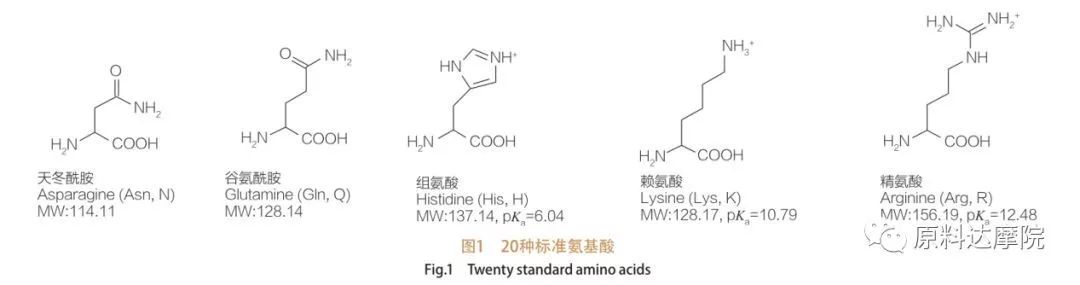
I-AAS ingachazwa njengekilasi lama-surfinents aqukethe ama-amino acid aqukethe amaqembu e-amino acid (ho 2 c-Ch-NH 2) noma izinsalela ze-amiino acid (Ho 2 C-Ch-NH- NH- NH Izindawo ezi-2 ezisebenzayo zama-amino acid zivumela ukusulwa kwezinhlobonhlobo ze-surffactants. Inani lama-acid ajwayelekile angama-20 ajwayelekile ama-acid aziwa ukuthi akhona emvelweni futhi abhekene nakho konke ukusabela okungokomzimba emisebenzini yokukhula kanye nemisebenzi yempilo. Ziyahlukahlukahlukana kuphela ngokusho kwensalela R (Umdwebo 1, Pk A yi-logarithm engemihle ye-dislociation ye-acid njalo yekhambi). Ezinye zingezona i-non-polar ne-hydrophobic, ezinye ziyizikhala ze-polar ne-hydrophilic, ezinye ziyisisekelo kanti ezinye zinama-acidic.
Ngoba ama-amino acid angamakhompiyutha avuselelekayo, ama-surfactants anTresession aqhamuka kuma-amino acid nawo anamandla aphezulu okuzinza futhi anobungane bemvelo. Isakhiwo esilula nesingokwemvelo, ubuthi obuphansi kanye ne-biodegrade okusheshayo kuvame ukubenza baphakame kakhulu kube yimali evamile. Kusetshenziswa izinto zokusetshenziswa kabusha ezivuselelekayo (isib. Ama-amino acid namafutha zemifino), i-AAS ingakhiqizwa ngemizila ehlukene ye-biotechnological kanye nemizila yamakhemikhali.
Ekuqaleni kwekhulu lama-20, ama-amino acid atholwa okokuqala ukuthi asetshenziswe njengezigatshana zokwenziwa kwama-surfinants.I-AAS yayisetshenziswa ikakhulukazi njengezilondolozi ezikwakhekeni kwamakhambi kanye nezimonyo.Ngaphezu kwalokho, kwatholakala ukuthi i-AAS isebenza ngokumelene namagciwane ahlukahlukene angela izifo, ama-tumors namagciwane. Ngo-1988, ukutholakala kwezimali ezibiza kakhulu ze-AAS ezikhiqizwayo emsebenzini ongaphezulu. Namuhla, ngokuthuthukiswa kwe-biotechnology, amanye ama-amino acid nawo akwazi ukuguqulwa ngokwezentengiselwano ngezinga elikhulu ngemvubelo, efakazela ngokungaqondile ukuthi ukukhiqizwa kwe-AAS kunobungani bemvelo.
Ukuthuthukiswa kwama-amino acid
Kusukela ekuqaleni kwekhulu le-19, lapho kwenzeka ngokwemvelo ama-amino acid acijile, izinhlaka zawo zabikezelwa ukuba zibaluleke kakhulu - zisebenziseka njengezinto zokulungiswa zokulungiswa kwama-Amphisile. Ucwaningo lokuqala ngokuhlanganiswa kuka-AAs lubikwa nguBondi ngo-1909.
Kulolo cwaningo, i-n-acylllsine ne-n-acylalanine zethulwe njengamaqembu e-hydrophilic ngenxa yama-surdophilic. Umsebenzi olandelayo uhilela ukuhlanganiswa kwama-lipoomino acid (AAS) usebenzisa i-glycine ne-alanine, kanye ne-hentrich et al. ushicilele uchungechunge lokutholile,Kubandakanya uhlelo lokusebenza lwe-patent lokuqala, ekusetshenzisweni kwe-Acyl Sarcosite nosawoti obukhona be-ACYL njengama-surfactants emikhiqizweni yokuhlanza indlu (isib. Ama-Shampoos, okokuhlanza kanye namazinyo).Kamuva, abacwaningi abaningi baphenya ukuhlanganiswa kanye nezakhiwo ze-physichicochemical of acyl amino acid. Kuze kube manje, kushicilelwe umzimba omkhulu wezincwadi ku-synthesis, izakhiwo, izinhlelo zezimboni kanye ne-biodeglamability of AAS.
Izakhiwo zesakhiwo ezingama-02
Amaketanga we-HYDROPOBIC we-Non-Polar Hydrophobic ama-AID we-AAS angahluka ngesakhiwo, ubude be-chain nenombolo.Ukwehlukahlukahluka kwesakhiwo kanye nomsebenzi omkhulu we-AAS uchaza ukwahlukahlukana kwabo okubanzi okubanzi kanye ne-physichicochemical ne-biological Properies. Amaqembu ekhanda ama-AA as akhiwa ama-amino acid noma ama-peptides. Umehluko emaqenjini ekhanda unquma i-adsorption, okuhlangene kanye nomsebenzi wezinto eziphilayo kwalezi zinto ezisetshenziswayo. Amaqembu asebenzayo eGroup Group bese enquma uhlobo lwe-AAS, kufaka phakathi i-cationic, anionic, enion, ne-amphoteric. Inhlanganisela yama-hydrophilic amino acid kanye nama-hydrophobic amakhethini amade we-hydrophobic akha isakhiwo se-amphiphilic esenza i-molecule ibe yindawo esebenzayo. Ngaphezu kwalokho, ukuba khona kwama-athomu e-asymmetric carbon ku-molecule kusiza ukwakha ama-molecule e-chiral.
03 Ukwakheka kwamakhemikhali
Onke ama-pettides nama-polypepdedes yimikhiqizo ye-polymerization yale minyaka engama-20 α-amaprotheni ama-acid. Yonke i-20 α-amino acid acid iqukethe iqembu le-carboxylic acid acid (-Cooh) kanye neqembu le-Amino elisebenzayo (-nh 2), zombili ezinamathiselwe kwi-tetrahedral α-carbon athomu. Ama-amino acid ahlukile komunye nomunye ngamaqembu ahlukene ama-R anamathele kwi-α-carbon (ngaphandle kwe-lycine, lapho iqembu le-rydrogen.) Amaqembu r angahluka ngesakhiwo, usayizi kanye ne-acidity (acidity, alkalinity). Lo mehluko futhi unquma i-solubility yama-amino acid emanzini.
Ama-amino acid angama-chiral (ngaphandle kwe-glycine) futhi akhuthele ngokwemvelo ngoba anokunye okuhlukile okuxhumene nekhabhoni ye-alpha. Ama-amino acid anezimo ezimbili ezingenzeka; Ziyizithombe ezingezona ezingezinyongezo zomunye nomunye, naphezu kweqiniso lokuthi inani lama-l-stereosomers liphakeme kakhulu. I-R-Group ekhona kwamanye ama-amino acid (Phyylalanine, i-tyrosine kanye ne-tryptophan) yi-aryl, eholela ekutholeni okuphezulu kwe-UV ku-280 nm. I-acidic α-cooh kanye ne-α-NH 2 e-Amino Acid iyakwazi uku-Ionization, futhi womabili ama-stereoisomers, noma ngabe kukhona, akha ukulingana kwe-ionization okuboniswe ngezansi.
R-cooh ↔r-coo-+ H+zela
R-nh3+zela↔r-NH2+ H+zela
Njengoba kukhonjisiwe ekulinganiseni kwe-Ionization ngenhla, ama-amino acid aqukethe okungenani amaqembu amabili abuthakathaka acidic; Kodwa-ke, iqembu le-carboxyl lilingana kakhulu i-acidic ngokuqhathaniswa neqembu le-amino elihlosiwe. I-PH 7.4, iqembu le-carboxyl lincipha ngenkathi iqembu le-amino lilawulwa. Ama-amino acid anamaqembu angewona angenakusebenza athathi athathi hlangothi kagesi kule mpilo kanye ne-zutterion.
04 Ukuhlukaniswa
I-AAS ingahlukaniswa ngokwezindlela ezine, ezichazwe ngezansi.
4.1 ngokuya ngemvelaphi
| Ngokuya ngemvelaphi, i-AAS ingahlukaniswa izigaba ezi-2 ngokulandelayo. ① isigaba semvelo Ezinye zezemvelo ezenzeka ngokwemvelo eziqukethe ama-amino acid nazo zinamandla okunciphisa ukwehla kwemvelo / ukungezwani kwezingxenyana, kanti ezinye zize zidlule ukusebenza kwe-glycolipids. Lezi aas zaziwa njenge-lipopeptides. I-Lipopeptides iyizinhlanganisela zesisindo eziphansi zamangqamuzana, ezivame ukukhiqizwa izinhlobo ze-bacillus.
I-AAS enjalo ihlukaniswe izigaba ezi-3 ze-subclass:I-Sursfactin, iTurin ne-Fengycin.
|
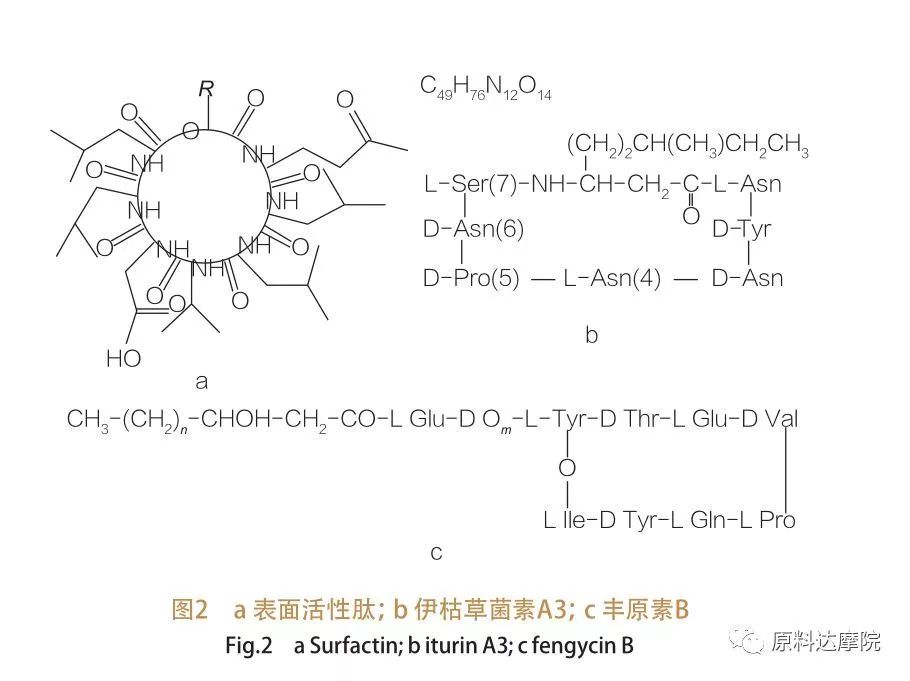
| Umndeni wama-peptides angaphezulu komhlaba uhlanganisa ama-heptapeptide ahlukahlukene wezinto ezahlukahlukene,Njengoba kukhonjisiwe kuMdwebo 2A, lapho i-C12-C12-C16 i-Acid Chain exhunyiwe exhunyiwe ku-peptide. I-peptide engaphezulu-esebenzayo yi-macrocyclic lactone lapho indandatho ivalwe khona yi-catalysis phakathi kwe-C-terminus ye-C-Cheddroxy Fatty Acid kanye ne-peptide. Esikhathini esingezansi se-iTurin, kukhona okuhlukahlukene okuyisithupha, okungukuthi i-Iturin A no-C, MyCoSubtilin kanye ne-bacillomycin d, f no-L.Kuzo zonke izimo, ama-heptapeptides axhumene namaketanga we-C14-C17 ama-β-amino fatty acids (amaketanga angahluka). Endabeni yama-Ekurimycins, iqembu le-amino endaweni ye-β-isikhundla lingakha isibopho se-Amide nge-C-terminus ngale ndlela eyakha isakhiwo se-macrocyclic ye-lacrocyclic.
I-subclass Fengycin iqukethe uFangycin A no-B, okubizwa nangokuthi i-Plipastatin lapho iTyr9 ilungiselelwe.I-devapettide ixhunyaniswe ne-C14 -C18 yagcwala i-acidy noma engafakwanga i-β-hydroxy fatty chain. Ngokuhlelekile, i-Plipastatin futhi i-macrocyclic lactone, equkethe i-tyr side chain endaweni ukulandelana kwe-peptide futhi yakha isibopho sangaphakathi se-c
② Isigaba sokwenziwa I-AAS nayo ingahle isetshenzisweni ngokusebenzisa noma yiliphi i-acidic, eyisisekelo futhi engathathi hlangothi ama-amino acid. Ama-amino acid ajwayelekile asetshenziselwa ukuhlanganiswa kwe-AAS yi-glutamic acid, i-serine, i-proline, i-aspartic acid, i-glycine, i-arginine, i-alanine, i-protein hydrolelotes. Le subclass of surfactants ingalungiswa ngamakhemikhali, enzymatic izindlela, nezindlela ze-chemoeenzymatic; Kodwa-ke, ngokukhiqizwa kwe-AAS, ukuhlanganiswa kwamakhemikhali kunokwenzeka ngokwezezimali. Izibonelo ezijwayelekile zifaka i-N-Lauroyl-L-Glutamic Acid ne-N-Palmicl-L-Glutamic Acid.
|
4.2 Kususelwa ekuhlolweni kwe-aliphatic chain substitunts
Kususelwa ekuhlolweni kwe-Aliphatic Chain, ama-Surfactants asuselwa ku-amino acid angahlukaniswa ngezinhlobo ezi-2.
Ngokusho kwesikhundla sabakwa-Persctituent
| ①N-esikhundleni se-AAS esikhundleni Ezihlanganisweni ezifakwa esikhundleni se-N, iqembu le-Amino lithathelwa indawo yiqembu le-lipophilic noma iqembu le-carboxyl, okuholele ekulahlekelweni okuyisisekelo. Isibonelo esilula kunazo zonke se-N-AAS esikhundleni se-N-Acyl Amino Acid, okuyizinto ezisetshenziswayo ze-anionic. I-N-esikhundleni se-AAS efakwe esikhundleni ine-pondide enamathiselwe phakathi kwezingxenye ze-hydrophobic ne-hydrophilic. I-Amide Bond inamandla okwenza isibopho se-hydrogen, esisiza ukucekelwa phansi kwalesi siphetho endaweni ene-acidic, ngaleyo ndlela ikwenze kube yinto engathandeki.
②C-esikhundleni se-AAS Ezinhlanganisweni ezibuyiselwe e-C, ukufakwa esikhundleni kwenzeka eqenjini leCarboxyl (nge-Amide noma i-Ester Bond). Amakhompiyutha ajwayelekile e-C-esikhundleni se-C (isib. Ama-esters noma ama-Alides) empeleni asetshenziswa ama-cotics.
③N- ne-C-esikhundleni se-AAS Kulolu hlobo lwe-survinant, zombili amaqembu e-amino ne-carboxyl yingxenye ye-hydrophilic. Lolu hlobo empeleni i-Amphoteric Surpfactant. |
4.3 Ngokuya ngenombolo yemisila ye-hydrophobic
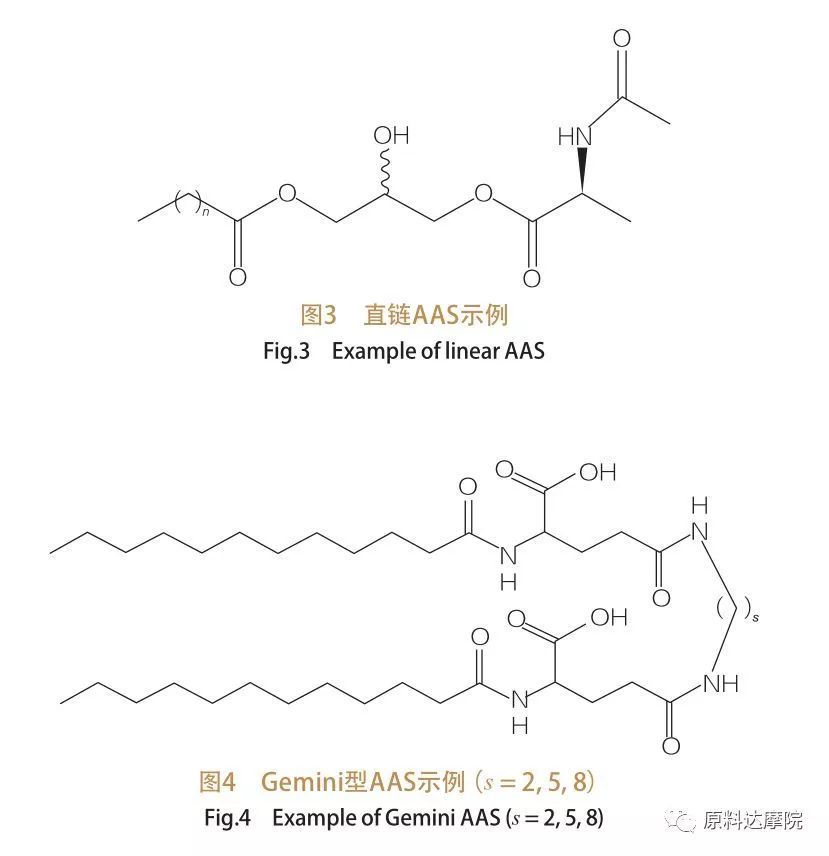
Ngokusekelwe kwinani lamaqembu ekhanda nemisila ye-hydrophobic, i-AAS ingahlukaniswa ngamaqembu amane. I-AAS eqondile ye-aas, i-Gemini (dimer) yohlobo lwe-AAS, uhlobo lwe-glyceceolipid aas, kanye ne-bicephalic Amphiphilic (BOLA) TYPE AAS. Ama-surfactants aqondile aqondile anama-surfactants aqukethe ama-amino acid anomsila owodwa we-hydrophobic (umdwebo 3). Uhlobo lwe-Gemini aas lunamaqembu amabili e-amino acid popular amaqembu kanye nemisila emibili ye-hydrophobic nge-molecule ngayinye (Umdwebo 4). Kulolu hlobo lwesakhiwo, ama-aas amabili aqondile axhumene axhumene ndawonye nge-spacer futhi ngenxa yalokho abizwa ngokuthi ama-dimers. E-glycerolipid aas, ngakolunye uhlangothi, imisila emibili ye-hydrophobic inamathiselwe eqenjini elifanayo le-amino acid head. Lezi zinto ezisetshenziswayo zingabhekwa njenge-analog ye-monoglycerides, i-diglycerides nama-phospholipids, ngenkathi ku-bola-hlobo aas, amaqembu amabili ekhanda ama-amino acid axhumene nomsila we-hydrophobic.
4.4 Ngokuya ngohlobo lweqembu lekhanda
①CAMICIC AAS
Iqembu lekhanda lalolu hlobo lwe-surviant linecala elihle. I-cationic aas yasekuqaleni i-ethyl cocoyl iyaxabana, okuyi-pyrlolidone carboxylate. Izakhiwo eziyingqayizivele nezihlukeyo zalokhu kusebenza kwenza kube wusizo ezimweni ze-magciwane, ama-antimicrobial agents, ama-antistatic agents, ama-hair conditioners, kanye nokuba mnene emehlweni nasekweshweni nasekuhambeni kalula. I-Singtere ne-mhatre yahlela i-arginine-based cationic AAS futhi ihlole izakhiwo zazo ze-physicochemical. Kulolu cwaningo, bathi izivuno eziphakeme zemikhiqizo etholakele kusetshenziswa imibandela yokusabela ye-baman-baumann. Ngobude obukhulayo we-ALKYL kanye ne-hydrophophobicity, umsebenzi we-surdophopobity watholakala ukuthi wanda futhi i-minelle yokuhlushwa (CMC) yokunciphisa. Enye iprotheni ye-acyl ye-quaternary, evame ukusetshenziswa njenge-conditioner emikhiqizweni yokunakekelwa kwezinwele.
②anionic AAS
Ku-anionic surffactants, iqembu lekhanda le-polar le-surving linecala elibi. ISarcosine (CH 3 -NH-Ch 2 -Cooh, N-Huthylglycine), i-amino acid etholakala ngokujwayelekile ema-urchins olwandle nezinkanyezi zasolwandle, ihlobene namakhemikhali e-glycine (NH 2 -COOH,), i-amino acid eyisisekelo etholakala kumaseli we-mammalian. -Cooh,) ihlobene namakhemikhali ku-glycine, okuyi-amino acid eyisisekelo etholakala kumaseli wezilwane ezincelisayo. I-Lauric acid, i-tetradecanoic acid, i-oleic acid kanye nama-haldes abo kanye nama-esters ajwayele ukusetshenziselwa ukuhlanganisa ama-surcosite arcuctants. ISarcosinates inobumnene ngokwemvelo ngakho-ke ivame ukusetshenziswa emlonyeni, ama-shampoos, amagwebu okushefa, ama-sunscreens, izihlanza zesikhumba, neminye imikhiqizo yezimonyo.
Amanye ama-anionic aas atholakalayo okuthengiswayo afaka i-Ampioft CS-22 ne-AmiliteGCK-12, okuyigama lokuhweba le-sodium n-cocoyl-l-glutamate ne-potassium n-cocoyl glyciate, ngokulandelana. I-Amilite isetshenziswa kakhulu njenge-ejenti ye-foaming, okokuhlanza, i-solubilizer, emulsifier kanye ne-dispsant, futhi inezicelo eziningi, ama-swampos, izinkuni zobuso, izinsipho zokuhlanza, izihlanza zelensi. I-Amisoft isetshenziswa njengesikhumba esimnene kanye nesihlanza sezinwele, ikakhulukazi ezihlanjululweni zobuso nakwimizimba, ukuvimba kokuhlanza okwenziwe, imikhiqizo yokunakekelwa komzimba, ama-shampoos kanye neminye imikhiqizo yokunakekelwa kwesikhumba.
③Zwitterionic noma ama-Amphoteric AAs
Ama-surphectants we-Amphoteric aqukethe amasayithi a-acidic ne-ayisisekelo futhi angashintsha ukushaja kwawo ngokushintsha inani le-PH. E-Alkaline Media baziphatha njengezindawo ezisetshenziswayo ze-anionic, ngenkathi ezindaweni ezinama-acidic ziziphatha njengabalingani be-cotics nabezindaba ezingathathi hlangothi njengama-amphoteric. I-Lauchl Lysine (LL) ne-Alkoxy (2-Hydroxyproppy) i-Argine yiwona kuphela ama-Amphoteric Surfactants atholakala kuma-amino acid. I-LL ingumkhiqizo wokuvuselela we-lysine ne-lauric acid. Ngenxa yesakhiwo sayo se-amphoteric, i-LL iyi-infoluble cishe kuzo zonke izinhlobo ze-solvents, ngaphandle kwe-alkaline noma i-solvents e-acidic. Njenge-organic powder, i-LL inama-adhesion amahle kakhulu ezindaweni ezinama-hydrophilic kanye ne-coefficient ephansi yokuxabana, enikeza le mandla elihle kakhulu lokuthambisa. I-LL isetshenziswa kabanzi kumakhilimu wesikhumba nakuma-conditioners wezinwele, futhi nayo isetshenziswa njenge-lubricant.
④Nonionic AAS
Ama-surfactants ase-nonion abonakala ngamaqembu ekhanda asePolar ngaphandle kwamacala asemthethweni. Ama-surfactants amasha ayisishiyagalombili ase-etheoonyated alungiswe ngu-Al-Sabagh et al. Kusuka ku-oyela-anicid α-amino acid. Kule nqubo, uL-phenyylalanine (lep) ne-L-EUCINE okokuqala kwaqalwa nge-hexadecanol, kulandelwa ukuhlaselwa nge-palmitic acid ukunikeza ama-anaides amabili nama-esters amabili ama-anicino acid. Izingadi kanye nama-esters babe sebekezele ukuguquguquka kwe-ethylene oxide ukulungiselela ama-phenenylalanine ama-derivativeves amathathu anezinombolo ezahlukahlukene zama-polloxyethylene Units (40, 60 no-100). Lezi aas ezingezona zitholwe zithola ukungcola okuhle nezinto ezigwebu.
05 Synthesis
5.1 Umzila oyisisekelo wokwenziwa
Ku-AAS, amaqembu e-hydrophobic anganamathiselwa kumasayithi a-Amine noma ama-carboxylic acid, noma emaketangeni aseceleni ama-amino acid. Ngokusekelwe kulokhu, kutholakala izindlela ezine eziyisisekelo zokwenziwa, njengoba kukhonjisiwe kuMfanekiso 5.
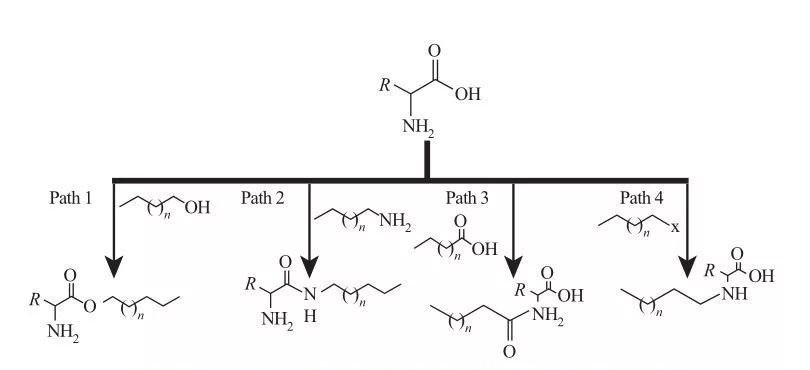
I-Fig.5 Isisekelo Sokuhlobana Syntherants of Amino Acid-based Surfice
| Indlela 1. I-Amphiphilic Ester Antines ikhiqizwa ngokusabela okubonakalayo, lapho i-surfication synthesis evame ukufinyelelwa ngokubonisa utshwala onamafutha nama-amino acid phambi kwe-ejenti yokuphelelwa ngamandla kanye ne-acidic detalyst. Kokunye ukuphendula, i-sulfuric acid isebenza njenge-catalyst kanye ne-ejenti yokuphelelwa ngamandla.
Indlela 2. I-Amino Acid ecushiwe esebenza ngama-alkylamines ukwakha amabhondi amide, okuholela ekuhlolweni kwama-ampiphilic we-amphiphilic.
Indlela 3. Ama-Amido acid ahlanganiswe ngokuphendula amaqembu a-Amine ama-amino acid ngama-amido acid.
Indlela 4. I-Alici Acid ende ye-ALKYLYL ihlanganiswe ukusabela kwamaqembu a-Amine nge Haloalalkanes. |
5.2 ukuthuthuka ku-synthesis kanye nokukhiqizwa
5.2.1 Ukuhlanganiswa kwama-amino acid / ama-surpide ama-surpide
I-N-Acyl noma i-O-acyl amino acid noma ama-peptides ingahle isetshenzisweni ngokuhlonya kwe-enzyme-catalyzed of anine noma amaqembu we-hydroxyl nama-fatty acids. Umbiko wokuqala we-solvent-free lipase-catalyzed synthesis we-amino acid amide noma i-methyl ester derivatives wasebenzisa uCandida Antarctica, ngezithelo ezisukela ku-25% kuya ku-90% ngokuya ngethagethi ye-amino acid. I-methyl ethyl ketone nayo isetshenziswe njenge-solvent kokunye ukusabela. Venderhagen et al. Ibuye ichaze ama-lipse kanye ne-proteines-catalyzed N-acrylition reactions yama-amino acid, ama-protein hydrolysteates kanye / noma okususelwa kwazo kusetshenziswa ingxube yamanzi kanye ne-organic solvents (isib.
Ezinsukwini zokuqala, inkinga enkulu ngokuhlanganiswa kwe-enzyme-catalyzed of AAS kwakuyizithelo eziphansi. Ngokusho kwe-Valivety et al. Isivuno se-n-tetradecanoyl amino acid derivatives sasingu-2% -10% ngisho nangemva kokusebenzisa ama-lipases ahlukene futhi sifaka ku-70 ° C izinsuku eziningi. Montet et al. Futhi uhlangane nezinkinga eziphathelene nesivuno esiphansi sama-amino acid ekuhlolweni kwe-N-Acyl lysine besebenzisa ama-fatty acids namafutha zemifino. Ngokusho kwabo, isivuno esikhulu somkhiqizo sasingama-19% ngaphansi kwezimo ezingenamahhala ze-solvent futhi zisebenzisa izixazululo ze-organic. Inkinga efanayo yahlangabezana ne-Valivety et al. Ku-synthesis we-N-CBZ-L-LYSIne noma i-N-CBZ-Lysine Methyl Ester Derivatives.
Kulolu cwaningo, bathi isivuno sika-3-o-tetradecanoyl-l-serine sasingama-80% lapho sisebenzisa i-serine evikelwe yi-n-novozyme 435 njenge-catalyst endaweni ebilisiwe ye-solvent-free emveni. UNagao noKito bafundze i-O-aryylition ye-L-Serine, i-L-Hoosererine, i-L-Threenine ne-L-tyrosine (ake) lapho bethola khona izithelo zokuqapha ze-L-HAMOSERIINE ne-L-SERINE zaziphansi, ngenkathi kungekho ukuhlakanipha kwe-l-threenine futhi kwenzeke.
Abaphenyi abaningi baye basekela ukusetshenziswa kwezindawo ezingabizi kakhulu ezitholakala kalula zokuhlanganiswa kwe-AAS esebenza ngezindleko. Soo et al. Kuthiwa ukulungiswa kwama-surfactants asuselwa ePalm Amafutha asebenza kahle nge-lipoenzyme engabonakali. Baphawule ukuthi isivuno semikhiqizo singangcono naphezu kwesikhathi sokudla (izinsuku eziyi-6). UGerova et al. Uphenya ukuhlanganiswa kanye nomsebenzi we-chiral n-palmittoyl aas asuselwa ku-methtionine, i-proline, e-threenine, i-phenenelalanine, i-phenyllycine engxube ye-cyclic / ye-racisec. UPang noChu bachaze ukuhlanganiswa kwama-monomes ancike ama-amino acid kanye nama-dicarboxylic acid ancike kwisixazululo uchungechunge lwama-pollamide asuselwa ku-amino acid acid abino ac ac ac astension ahlelwe yi-Amino acid acid acid acid acid acid-acid amino acid acid acid acid acid acid acid acid-based amino acid acid acid acid-acid amio acid acid acid acid-based amino acid acid-based amino acid acid acid acid abici acid acid acid-based amino acid acid acid acid acid acid acid acid acid acid-acid abici acid acid acid-based ama-pollamide esters ahlelwe yimidlalo yokuvumelanisa.
UCantaeuzene noGuerreiro babike ukucaciswa kwamaqembu e-carboxylic acid we-boc-aha-oh ne-boc-ask-ask-ask-oh nge-diolt-ancy alingise Kulolu cwaningo, ukusabela kwe-BOC-ALA-OH enotshwala onamafutha kuze kube yi-16 Carbons kwanikeza isivuno esihle (51%), ngenkathi i-BOC-ASS Carbons yayingcono, ngesivuno esihambisanayo sangama-63%. 99.9%) Kuvunyelwa kusuka kuma-58% kuya ku-76%, ahlelwe ukwakheka kwama-amide ama-alkyyalines noma ama-ark-arg-ome anamafutha anamafutha nge-CBZ-Org-Ome, lapho i-papain-iome ibangelwa yi-catalyst.
5.2.2 Ukuhlanganiswa kwe-Amino Acid / Ama-Supredi Fino Acid
Ama-Surfice asuselwa ku-Amino acid Gemini aqukethe ama-molecule amabili aqondile aas axhumene axhumeke ekhanda ngekhanda ngeqembu le-spacer. Kunezinhlelo ezi-2 ezingenzeka zokuhlanganiswa kwe-chemoeenzymatic ze-Gemini-Type Amino Acid-based Surficid-based Surficid (Figure 6 no-7). Ku-Figure 6, 2 Ama-Amino acid derivatives asabele ngenhlamba njengeqembu le-spacer kwathi kwethulwa iqembu lama-2 hydrophobic. Ku-Figure 7, izakhiwo ezi-2 eziqondile ze-chain zixhunyaniswe ngokuqondile yiqembu le-BIFUNGAAL Spacer.
Ukuthuthukiswa kokuqala kwe-enzyme-catalyzed synthesis ye-Gemini Liponomino Acids yaphayona nguValivety et al. Yoshimura et al. Uphenya ukuhlanganiswa, ama-adsorption kanye nokuhlanganiswa kwe-amino acid-based Gemini Surffactant esekelwe kwiCystine ne-N-Alkyl Bromide. Ama-symelactants ahlanganisiwe aqhathaniswa ne-monomeric sursuracial ehambisanayo. Faustino et al. Ichaze ukuhlanganiswa kwe-anionic urea-based monomeic aas ngokusekelwe ku-L-CYSTINE, D-CYSTINE, l-cystine, i-l-sulfoine, i-l-sulfoine, i-l-sulfoine kanye ne-veall-cysteine, i-veary-state fluoreslit yabo. Kwakhonjiswa ukuthi inani le-CMC le-GEMINI laliphansi ngokuqhathanisa i-monomer ne-gemini.
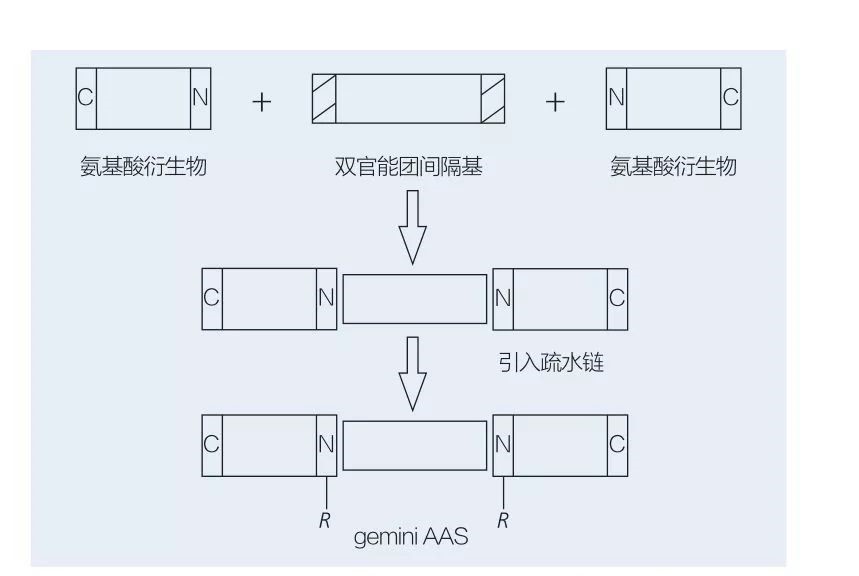
I-Fig.6 Synthesis of Gemini AAS usebenzisa i-AA Derivatives ne-Spacer, kulandelwe ukufakwa kweqembu le-hydrophobic
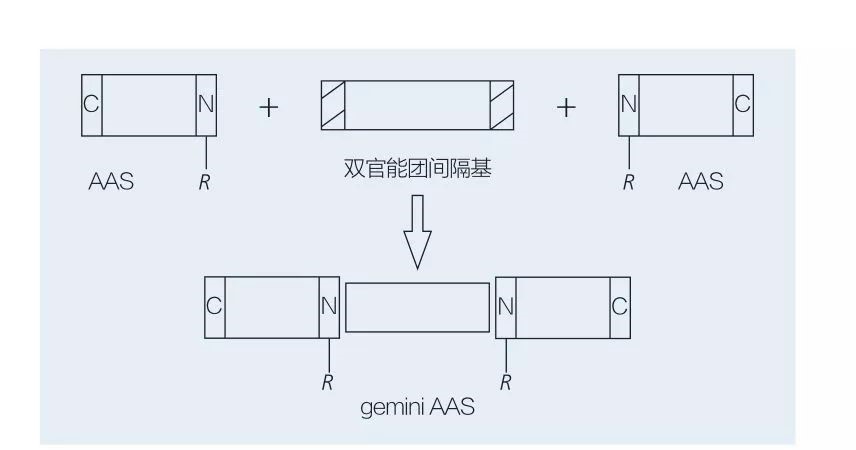
I-Fig.7 Synthesis of Gemini AASH usebenzisa i-Bifunctional Spacer ne-AAS
5.2.3 Ukuhlanganiswa kwe-glycececececeopid amino acid / ama-peptide surfactants
I-Glycececeolipid amino acid / ama-surtide surfide ayisigaba esisha se-lipid amino acid analogs ye-Glycerol Mono- (noma di-) ama-phospholipids, ngenxa yesakhiwo sawo se-amino acid exhunywe esihlokweni se-glycel nge-ester bond. Ukuhlanganiswa kwalezi zinto ezisetshenziswayo ziqala ngokulungiswa kwama-glycerol esters ama-amino acid kumazinga okushisa aphakeme nasebukhoneni be-catalyst ye-acidic (isib. BF 3). I-enzyme-catalyzed synnesis (usebenzisa ama-hydrolases, ama-proterises kanye nama-lipases njengama-catalysts) futhi kuyindlela enhle (Umdwebo 8).
I-enzyme-catalyzed synthesis ye-Artinined arginecerides ahlanganisa ama-patain sekubikiwe. Ukuhlanganiswa kwe-Diacylllyclycerol Ester conjugates kusuka ku-acetylarginine kanye nokuhlaziywa kwezindawo zabo ze-physicochemical kuye kwabikwa.
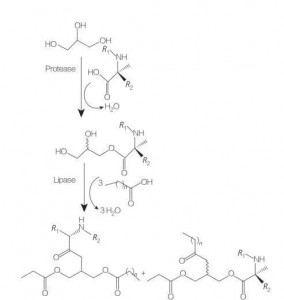
Umkhiwane we-synthesis we-mono ne-diacyllycel amino acid conjugates
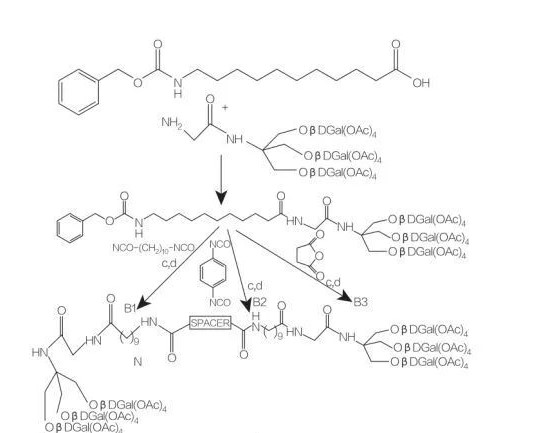
spacer: nh- (ch2)10-Nh: compoundb1
I-Spacer: NH-C6H4-Nh: compoundb2
spacer: ch2-Cha2: compoundb3
I-Fig.9 Synthetris of Symmetric Amphipheris ezisuselwa ku-Tris (HydroxyMethyl) Aminomethane
5.2.4 Ukuhlanganiswa kwe-Amino Acid / Ama-Peptide Surfactants
I-Amphipheriles esekelwe e-Amino acid-acid aphphipheriles iqukethe ama-2 amino acid axhumeke kuchungechunge olufanayo lwe-hydrophobic. UFranceschi et al. kuchaze ukuhlanganiswa kwama-amphiphiles weBola-Type Amphiphiles ngama-2 amino acid (d- noma l-alanine noma i-l-hestidine) kanye ne-1 alkyl chain yobude obuhlukile futhi baphenya umsebenzi wabo ohlukile. Baxoxa ngokuhlanganiswa kanye nokuhlanganiswa kwenoveli bola-Type Amphiphiles nge-Amino Acid Fraction nge-Amino Acid Fraction (kusetshenziswa noma iyiphi i-acid engajwayelekile β-amino noma i-C12 -C -c20 Spacer Group. Ama-acid ajwayelekile ama-β-amino asetshenzisiwe angaba ushukela i-aminoacid, i-azidothymin (AZT) -Dise-acid ye-amino acid, ne-Amino acid, kanye notshwala be-amino abasuselwa ku-AZT (Umdwebo 9). Ukuhlanganiswa kwe-Symmetrical Bola-Type Amphipheriles okususelwa ku-Tris (HydroxyMethyl) Aminomethane (Tris) (Umdwebo 9).
06 Izakhiwo ze-Physicochemical
Kuyaziwa ukuthi ama-surficial acid acid ahlukahlukene (AAS) ahlukahlukene futhi ahlukahlukene ngokwemvelo futhi akwazi ukusebenza ngezicelo eziningi ezinjengokuthula okuhle, ukusebenza okuphezulu komsebenzi, ukumelana nomsebenzi omkhulu).
Ngokusekelwe kwizakhiwo ezisetshenziswayo zama-amino acid (isb
6.1 Ukuhlushwa Okubalulekile Kwe-Micelle (CMC)
Ukuhlushwa okubucayi kwe-micelle kungenye yezingcawu ezibalulekile ze-surficiants futhi kulawule izindawo eziningi ezisebenzayo ezinjengokuthula, ukuqhubeka kwe-hydrophobity, kukhulisa ukuncipha kwenani le-hydrocarbon (njll. Ama-Surfactants asuselwa kuma-amino acid ajwayele ukuba namanani aphansi we-CMC ngokuqhathaniswa nama-surfactants ajwayelekile.
Ngezinhlanganisela ezahlukahlukene zamaqembu ekhanda kanye nemisila ye-hydrophobic (i-mono-cationic amide, i-bi-cationic amide, i-bi-cetioc amide-based ester), infante et al. Kwenziwe ama-AAS amathathu asuselwa ku-Argine-based futhi afundele i-CMC ne-γmpc (ukungezwani komhlaba (ukubhekana nokungezwani komhlaba ku-CMC), okubonisa ukuthi amanani we-CMC nama-γmc ancipha ngokukhulisa umsila we-hydrophobic. Kokunye ukuhlola, i-sangare ne-mhatre bathola ukuthi i-CMC ye-CMC ye-N-α-Accylarginine Surfactants yehle ngokwandisa inani lama-athomu omsila we-hydrophobic.
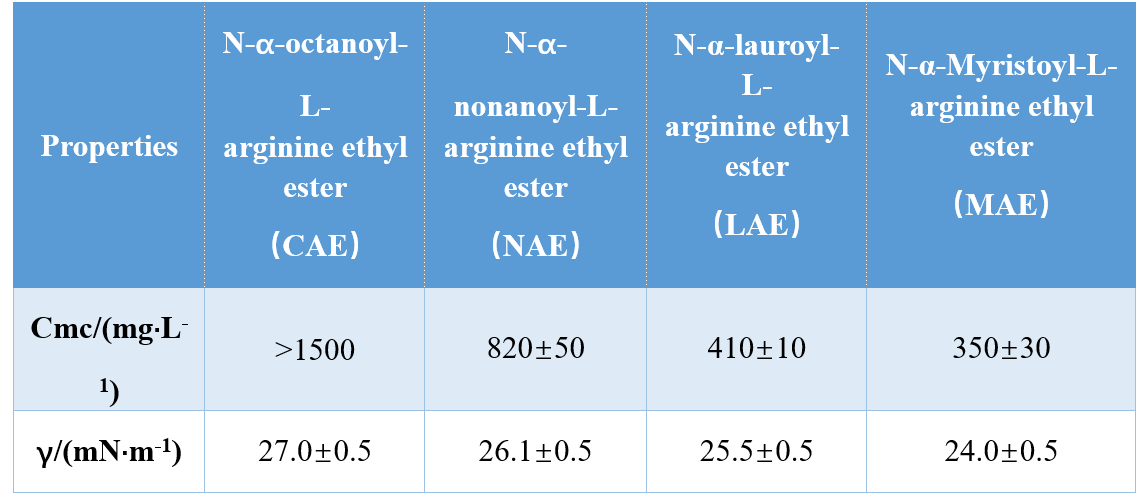
Yoshimura et al. Uphenya i-CMC ye-Cysteine-asuswe ama-Amino acid Gemini-based Gemini acid futhi abonise ukuthi ubude be-CMOBOUNCE buttle chain ku-14 kuye kwayi-14.
Faustino et al. Kubikwe ukwakheka kweMicellege Ehlanganisiwe ezixazululweni zamanzi zamanzi zama-antionic Gemini Surfactants asuselwa ku-Cystine. Ama-gemini surficactants nawo aqhathaniswa ne-monomentactive ehambelana ne-monomeric surcialic (C 8 CYS). Amanani we-CMC we-Lipid-Surfactant Mixtures kubikwa ukuthi aphansi kunaleyo yobunzima obumsulwa. I-Gemini Surfactants kanye ne-1,2-diheplanoyl-sn-glyceryl-3-phosphocholine, i-phospholipid engenamanzi, i-phospholipid ye-minelle, ine-cmc ezingeni lama-mollimolar.
ISrytha no-Aramaki baphenya ukwakheka kweMicelles ye-Viscoelastic Worm-efana nezixazululo zamanzi ezixubile ze-Amino acid-based antionic-antion antionic-an anionic-basesic surfic-antion sursuctants lapho usawoti wokuhlanganiswa. Kulolu cwaningo, i-n-dodecyl glutamate yatholakala ukuthi inokushisa okuphezulu kwe-krafch; Kodwa-ke, lapho ungathathi hlangothi nge-amino acid L-Lysine, kwakhiqizwa ama-miClelles futhi ikhambi laqala ukuziphatha njengoketshezi olungu-25 ° C.
6.2 I-solubity yamanzi enhle
I-solubility yamanzi enhle ka-AAS ingenxa yokuba khona kwamabhondi angeziwe we-CO-NH. Lokhu kwenza i-AAS ibe yi-biodegradable yemvelo futhi inobungani kunemvelo ehambelana nalokhu okuhambelana nawo. I-solubility yamanzi ye-N-Acyl-L-Glutamic acid ingcono kakhulu ngenxa yamaqembu awo ama-2 carboxyl. I-solubility yamanzi ye-CN (CN) futhi yinhle ngoba kunamaqembu ama-2 ama-Argine arginine kuma-molecule ayi-1, aphumela ekuxhumekeni kweseli nokuvinjwa kwamagciwane asebenza ngokugxila okuphansi.
6.3 krafft lokushisa lokushisa ne-krafft point
Ukushisa kwe-Krafft kungaqondakala njengokuziphatha okukhethekile kokusebenza kwe-surfactants ekwandisa ukusekwa kwemali ephakeme kakhulu ngenhla kwendlela ethile. Ama-Ionic Surfactants anokuthambekela kokukhiqiza ama-hydrate aqinile, angahle aphume emanzini. Emazingeni okushisa athile (okuthiwa yi-Kraft lokushisa), ukwanda okumangazayo nokwenqanyulayo kwe-solubity yama-surfactants kuvame ukubonwa. Iphoyinti le-krafft le-ionic surffantant lingamazinga okushisa we-krafft e-CMC.
Lesi sici se-solubity sivame ukubonwa ama-Ionic Surfactants futhi singachazwa kanjena: Ukubhidlizwa kwe-monomer yamahhala ye-Surfactant kunqunyelwe ngaphansi kwamazinga okushisa we-krafch kuze kube yilapho i-Krafft yayo kancane kancane ngenxa yokwakheka kwe-micelle. Ukuqinisekisa i-solubility ephelele, kuyadingeka ukulungiselela ukwakhiwa okushisayo emazingeni okushisa angenhla kwe-Krafft Point.
Ukushisa kwe-Krafft of AAS kufundwe kwaqhathaniswa nokuqhathaniswa ne-surfiftants evamile yokwenziwa.shrestha no-Aramaki bafunda indawo yokushisa ye-Agraffy ngo-2-5 × 10-6 mol-L -1 elandelwa yizinhlobo eziyisithupha ezihlukile ka-n-hexadecanoyl aas futhi waxoxa ngobudlelwano phakathi kwezinga lokushisa kwe-krafft kanye nezinsalela ze-amino acid.
Esivivinyweni, kwatholakala ukuthi izinga lokushisa le-krafft le-N-Hexadecanoyl aAs likhuphuke ngokuncipha kwezinsalela ze-amino acid (personthana Kwaphethwa kuthi ngazo zombili izinhlelo ze-Alanine nasePhenyLlalanine, ukusebenzisana kwe-DL kunamandla kunokuxhumana kwe-LL ngesimo esiqinile sosawoti we-N-Hexadecanoyl AAS.
Brito et al. Kunqume amazinga okushisa we-krafch ochungechunge oluthathu lwe-Amino Acid-based Surficimentry usebenzisa ukuskena okuhlukile kwe-microcalorimetry futhi kutholakale ukuthi ukuguqula i-ion ye-toodide kuholele ekuphumeni kwe-iodide kuphumela ekulweni okuphezulu kwe-Kraftting Cardrate (cishe ngo-6 Ukuba khona kwama-CIS-Double Bonds kanye nokungatholakali okukhona kuma-Ser-Derivatives amade aholele ekunciphiseni okukhulu emazingeni okushisa we-krafch. I-N-DoDecyl Glutamate kubikwa ukuthi ine-Krafch lokushisa eliphakeme. Kodwa-ke, ukungathathi hlangothi nge-Amino Acid L-Lysine kwaholela ekusungulweni kwama-Micelles ngesixazululo esiziphatha njengoketshezi lweNewtonian C.
6.4 ukungezwani komhlaba
Ukuxineka komhlaba kwezisulu ezihlobene kuhlobene nobude be-chain yengxenye ye-hydrophobic. Zhang et al. Unqume ukungezwani kwe-sodium cocoyl glycate nge-Wilhelmy Plate indlela (25 ± 0,2 0.2) ° C futhi wanquma inani lengxabano ye-CMC njenge-33 MN-M -1, CMC njenge-0.21 mmol-L -1. Yoshimura et al. Unqume ukungezwani okungaphezulu kwe-2c n cys Uhlobo lwe-amino acid esekelweni elingaphezulu komhlaba esekelwe ebusweni be-2c n cys asebekhulile abasebenzayo. Kutholakale ukuthi ukungezwani kwengxabano e-CMC kwehle ngokwandisa ubude be-chain (kuze kube yi-n = 8), ngenkathi inkambiso ibuyiselwe emuva ngenxa yobude be-n = 12 noma isikhathi eside.
Umphumela we-CAC1 2 ngokungezwani komhlaba we-dicarboxyled amino acid-based surfactants nawo afundwe. Kulezi zifundo, i-CAC1 2 yengezwa kwizixazululo zamanzi zamanzi ama-dicarbox hlobo luhlobo lwe-amino acid-uhlobo lwe-amino acid-hlobo ama-amino acid (C12 Malna 2, c12 aspna 2, kanye no-C12 Gluna 2). Amanani wethafa ngemuva kokuqhathaniswa kwe-CMC futhi kwatholakala ukuthi ukungezwani kwahle kwancipha ekugxileni okuphansi kakhulu kwe-CAC1 2. Lokhu kungenxa yomphumela we-calcium ion ehlelweni lwe-survictant esibonakalayo samanzi. Izingxabano zangaphandle zikasawoti we-n-dodecyalamalonate ne-n-dodecyastate, ngakolunye uhlangothi, zazingacishe zibe ngu-10 mmol-L -1 CAC1 2 okuhlushwa. Ngaphezulu kwe-10 mmol-L -1, ukungezwani kobuso kukhulisa kakhulu, ngenxa yokwakhiwa kwemvula yosawoti we-calcium we-survium. Ngosawoti we-disedium we-n-dodecyl glutamate, ukungezwa okulinganiselwe kwe-CAC1 2 kuholele ekunciphiseni okukhulu ekuxineni komhlaba, ngenkathi kuqhubeka ukukhuphuka ku-CAC1 2 Ukuhlushwa akusekhodale ushintsho olukhulu.
Ukunquma ama-kinetics e-adsorption we-Gemini-Type AAS esibonakalayo samanzi esibonakalayo, ukungezwani okuguquguqukayo kwanqunywa kusetshenziswa indlela ephezulu ye-Bubble Pressure. Imiphumela iveze ukuthi isikhathi eside sokuhlola isikhathi eside, i-2c 12 cys Dynamic Surmate Tension ayizange iguquke. Ukwehla kwengxabano engaphezulu enamandla kuncike kokuhlushwa, ubude bemisila ye-hydrophobic, nenombolo yemisila ye-hydrophobic. Ukwanda kokuhlushwa kwe-surfactant, ukwehla kobude bechungechunge kanye nenombolo yamaketanga kuholele ekuwohlokeni okusheshayo. Imiphumela etholwe ukugxila okuphezulu kwe-C N CYS (n = 8 kuye ku-12) isondele kakhulu ku-γ cmc elinganiswa yindlela ye-Wilhelmy.
Kokunye ukuhlola, ukungezwani okunamandla kwe-sodium dilauryl cystine (SDLC) kanye ne-sodium decamino cystine kunqunywe yindlela yeplanethi ye-wilhelmy, futhi ngaphezu kwalokho, ukungezwani komhlaba okulinganayo kwezixazululo zabo ezinamanzi kwanqunywa yindlela yokulahla yevolumu. Ukusabela kwama-Dislulfide Bonds kwaphinde kwaphenywa ezinye izindlela. Ukungezwa kwe-MercaPtoethanol kuya ku-0,1 mmol-L -1SDLC Isixazululo kuholele ekunyuseni okusheshayo kokungezwani komhlaba kusuka ku-34 m-m-1 kuye ku-53 MN-M -1. Njengoba uNaclo angakwazi ukwenza izibopho ze-dislulfide ze-SDLC ziye emaqenjini e-sulfonic acid, akukho zinhlangano ezihlanganisiwe lapho i-naclo (5 mmol-L -1) yengezwe ku-0,1 mmol-L-SDLC solution. Ukuhambisa i-Electron Microscopy kanye nemiphumela yokusakazeka yokukhanya okunamandla kukhombisa ukuthi akukho okuhlanganisiwe okwakhiwa kwikhambi. Ukungezwani kwe-surface ye-SDLC kwatholakala ukuthi kwanda kusuka kuma-34 m-m -1 kuya ku-60 m-m -1 esikhathini semizuzu engama-20.
6.5 Ukusebenzisana Okungaphezulu Kanambambili
Ezesayensi yempilo, amaqembu amaningi afunde izakhiwo ezishubile zokuxutshwa kwama-aas aas (diacyllycerol arganine arvine-based surfice) kanye nama-phospholipids esibonakalayo samanzi esibonakalayo, ekugcineni aphethe ukuthi le mpahla engekho kahle ibangela ukubambeka kokusebenzisana kwe-electrostatic.
6.6 Izakhiwo Ezihlanganisiwe
Ukusakazeka okukhanyayo okuvamile kuvame ukusetshenziswa ukuthola izakhiwo ezihlanganisiwe zama-monomers asuselwa ku-amino acid kanye nama-gemini ancishiswe ekugxileni ngenhla kwe-CMC, kuthela i-DHDrodynamic Dimelle dh (= 2r h). Ama-agregates akhiwa ngu-c n Cys kanye nama-2cn CYs amakhulu futhi anokusatshalaliswa okubanzi okuqhathaniswa namanye ama-surffactants. Zonke izinto ezisetshenziswayo ngaphandle kwama-2c 12 ama-CYs ngokuvamile ahlanganisa ama-10 NM. Usayizi we-Micelle oguqukayo we-Gemini Surfactants akhulu kakhulu kunaleyo yozakwabo abasebenza nge-monomeric. Ukwanda kobude be-hydrocarbon nakho kuholela ekwandeni kosayizi we-micelle. ohta et al. Ichaze izakhiwo ezihlanganisiwe zama-stereoisomers amathathu ahlukene we-n-dodecyl-phenyl-alanyl-phenyl-alanine tetramethylamonium ngesisombululo esinamanzi futhi akhombise ukuthi ama-diatendlaosomers aqukethe okugcwele okuhlangene kwikhambi lamanzi afanayo. Iwahashi et al. ephenywe yi-dichromism eyindilinga, i-NMR ne-Vapor Pressure Osmometry ukwakheka kwezinhlanganisela ze-chirali ze-n-dodecanoyl-l-glutamic kanye ne-tetrahydroforunan, i-acetonitrile, 1,4-dioxane kanye ne-1,2-dichlorohone) Ngezindawo ezijikelezayo kuphenywe yi-dichroism eyindilinga, i-NMR ne-Vapor Pressure Osmometry.
6.7 I-adsorption yokuxhumana
I-adsorption ye-adfacial ye-amino acid-based surficactants futhi ukuqhathanisa kwayo nomlingani wayo ojwayelekile nakho kungenye yezinkomba zokucwaninga. Isibonelo, izakhiwo ze-adsorption ze-adsorption ze-Dodecyl e-Acomatic Amico Acid etholakele kusuka ku-Lep aphenywe. Imiphumela ikhombisile ukuthi ake abonise izindawo ezisezingeni eliphansi zokuzijabulisa ephepheni legesi nasezibonisi zamanzi / nge-hexane, ngokulandelana.
I-Bordes et al. Uphenya ngokuziphatha kwesixazululo kanye ne-adsorption esibonakalayo samanzi esibonakalayo sama-dicarboxboxwed ama-amino acid ama-glutamate, i-dodecyl aspartate, kanye nama-ampinolalonate (nama-aninomalonate (ngama-3, 2, nama-atom e-carbon aphakathi kwamaqembu amabili e-carboxyl, ngokulandelana). Ngokusho kwalo mbiko, i-CMC ye-DICARBEXYLENED SURFACTIRESS yayisikhathi esingu-4-5 esiphakeme kunaleso sosawoti we-dodecboxyled dodecyledy. Lokhu kuthiwa kwakhiwe ukwakheka kwama-hydrogen Bonds phakathi kwama-dicarboxylieted surficatents kanye nama-molecule angomakhelwane ngokusebenzisa amaqembu amide.
6.8 Ukuziphatha kwesigaba
Izigaba ze-Isotropic cubic izigaba zibhekwa ngama-surfactants ngokugxila okuphezulu kakhulu. Ama-molecule asetshenzisiwe anamaqembu amakhulu ekhanda athambekele ekwakhekeni kwama-aggregates we-curvature emincane eyakhayo. UMarques et al. wafunda ngokuziphatha kwesigaba kwe-12lys12 / 12sser kanye ne-8lys8 / 16sser systems (bheka umfanekiso 10), kanti uhlelo lwe-vesicular lunesistimu ye-7lys8 / 16sser lubonisa isici esiqhubekayo (isizinda sesigaba se-micLellar phakathi kwesigaba esincane sesigaba se-micLellar kanye Isifunda se-Vesicle Phase). Kumele kwaziwe ukuthi ngesifunda se-vesicle sohlelo lwe-12lys12 / 12s, ama-vesicles ahlala ehlangana neMicelles, kanti isifunda se-vesicle sohlelo lwe-8lys8 / 16SOR sinama-vesicles kuphela.
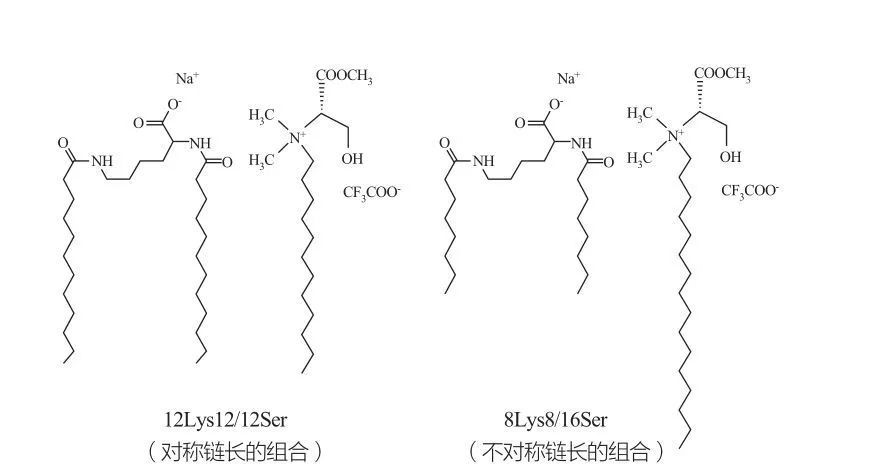
Ukuhlanganiswa kwe-catanionic kwe-lysine- kanye nama-syractants asuselwa ku-serine: symmetric 12lys12 / 12s Bart (kwesobunxele) kanye ne-asymmetric 8lys8 / kwesokudla)
I-6.9 ekhombisa amandla
UKouchi et al. Kuhlolisise amandla okuqinisa, ukungezwani, okungafani, nokubonakala kwe-n- [3-dodecyl-2-hydroxypropyl] -l-glutamate, namanye ama-aas. Ngokuqhathanisa nama-surfactics okwenziwa (abalingani babo abajwayelekile be-amphoteric kanye ne-amphoteric), imiphumela ikhombisile ukuthi ama-AAs anekhono elinamandla elinamandla kunabasebenzi abajwayelekile.
Baczko et al. I-synthesised Novel Anionic amino Acid Surfactants futhi waphenya ukufaneleka kwawo njengoba izixazululo ze-NMR ezenzelwe i-NMR ezenzelwe i-NMR. Uchungechunge lwe-Amphiphilic esekelwe e-Amphiphilic L-PHPE noma i-L-ALA Derivatives enemisila ehlukene ye-hydrophobic (i-Penting ~ tetradecyl) yahlanganiswa ngokuphendula ama-amino acid nge-O-Sulfobenzoic Anhydride. I-Wu et al. ahlanganiswe usawoti we-sodium we-n-tatty acyl aas futhiUphenya amandla abo e-emulsication emamugqeni wawo wamafutha, futhi imiphumela yabonisa ukuthi lezi zinto ezisetshenziswayo zenziwa kangcono nge-ethyl acetate njengesigaba samafutha kune-N-HEXAWE njengesigaba samafutha.
6.10 ukuthuthuka ku-synthesis kanye nokukhiqizwa
Ukumelana kwamanzi kanzima kungaqondakala njengekhono lokungasebenzi kwama-surfactants ukumelana nokuba khona kwe-ion njenge-calcium ne-magnesium emanzini anzima, okungukuthi, amandla okugwema ukwenela kwi-calcium insicu. Ama-Surfactants anokumelana kwamanzi aphezulu asiza kakhulu ekwakhiweni kwama-detergent kanye nemikhiqizo yokunakekelwa komuntu. Ukumelana kwamanzi kanzima kungahlaziywa ngokubala ushintsho ekuhlanyeniseni nasekusebenzeni komhlaba okuphelelwa yi-calcium ions.
Enye indlela yokuhlola ukumelana kwamanzi kanzima ukubala amaphesenti noma amagremu okuxhamazela okudingekayo kwe-calcium insipho eyakhiwe kusuka ku-100 g we-sodium oleate kuhlakazeke emanzini. Ezindaweni ezinamanzi afudumele aphezulu, ukugxila okuphezulu kwe-calcium kanye ne-magnesium ion kanye nokuqukethwe kwamaminerali kungenza ukuthi kube nzima izinhlelo zokusebenza ezisebenzayo. Imvamisa i-ion ion isetshenziswa njenge-counter ion ye-anionic ye-anionic yokwenziwa. Njengoba i-waclent calcium ion iboshelwe ama-molekyuli asetshenzisiwe womabili, kubangela ukuthi i-surfactant iqondise kalula kusuka kusisombululo enze detegency okungenzeka ukuthi kungenzeka.
Ucwaningo lokumelana kwamanzi okunzima kukhombisa ukuthi i-acid kanye nokumelana kwamanzi kanzima kwathonywa ngokuqinile yiqembu elengeziwe le-carboxyl, futhi i-acid kanye nokumelana kwamanzi okunzima kwanda ngokuqhubeka nokunyuka kobude beqembu le-carbox. Ukuhleleka kwe-acid kanye nokumelana kwamanzi kanzima kwakungu-C 12 glycine <c 12 plutamate <c 12 glutamate. Ukuqhathanisa ibhodi le-DicarboxBooxed Amide kanye ne-dicarboxyled amino survin, ngokulandelana, kwatholakala ukuthi uhla lwasemuva lwalubanzi futhi nomsebenzi waso ongaphezulu ukhuphuke ngokufakwa kwenani elifanele le-acid. Ama-Acid we-DICARBEBYELY N-ALKYL AIDICA akhombisa umphumela we-Chelating Effession phambi kwe-calcium ion, kanye no-C 12 aspartate kwakhiwa ijeli elimhlophe. I-C 12 glutamate ikhombise umsebenzi ophakeme we-caple can 2 + okuhlushwa okuphezulu futhi kulindeleke ukuthi isetshenziswe e-Seawater Desalination.
6.11 Ukuphazamiseka
Ukusabalalisa kubhekisele emandleni okuxhamazela ukuvikela ama-coalescence kanye nokudinwa kokuxhamazela kwisixazululo.Ukuhlakazeka kuyindawo ebalulekile ye-surficacters ebenza basebenzise ukusetshenziswa ezihlalweni, izimonyo kanye nemithi.Umenzeli osabalalisa kufanele aqukathe i-ester, ether, amide noma i-amino isibopho phakathi kweqembu le-hydrophobic kanye neqembu le-terminal hydrophilic (noma phakathi kwamaqembu aqondile we-hydrophobic).
Ngokuvamile, ama-anionic ama-surfactants anjenge-alkanolamido sulfates kanye nama-amphoteric surphectants anjenge-anidosulfobetaine asebenza ngempumelelo njengama-ejenti asabalalisa ama-calcium insipho.
Imizamo eminingi yocwaningo inqume ukungafani kwe-AAS, lapho kutholakala khona i-N-Lauroyl Lysine ukuthi ayihambelani kahle namanzi futhi kunzima ukuyisebenzisela ukwakheka kwezimonyo.Kulolu chungechunge, ama-amino acid angenamikhawulo angenamikhawulo anokuhlakazeka okuhle kakhulu futhi asetshenziswa embonini yezimonyo ukwenza ngcono ukwakhiwa.
07 Ubuthi
Ama-surfactants ajwayelekile, ikakhulukazi ama-cationic surfic, anobuthi kakhulu ezintweni eziphilayo zasemanzini. Ubuthi babo obuqine kungenxa yento yokuxhumana ye-adsorption-ion of surfications esibonakalayo samanzi. Ukunciphisa i-CMC yama-surfactants kuvame ukuholela ekutheni kube nokuqina kwe-adposicial adsorption yama-surfactants, okuvame ukuholela ebuthi bayo obukhulu. Ukwanda kobude be-hydrophobic chain of surffactants nakho kuholela ekwandeni kobuthi obuqine.Iningi le-AAS liphansi noma alinobuthi kubantu kanye nemvelo (ikakhulukazi ezintweni eziphilayo zasolwandle) futhi zilungele ukusetshenziswa njengezithako zokudla, amakhamishini kanye nezimonyo.Abaphenyi abaningi bakhombise ukuthi ama-surfactants we-Amino acid athambile futhi awacasuli esikhunjeni. Ama-surfactants asuselwa ku-Arginine ayaziwa ukuthi anobuthi obuncane kunabalingani babo abajwayelekile.
Brito et al. wafunda izakhiwo ze-amphiphiles ezisuselwa ku-amino acid kanye nama-verivatives avela ku-tyrosine (TRIVE), i-hydroxyproline (hys), i-lysine (i-lyr) kanye ne-lysine (i-hys) kanye ne-lysine (i-lyr) kanye ne-lysine (i-lyr) kanye ne-lysine (i-lyr) kanye ne-lysine (i-lyr) kanye ne-lysine (i-lyr) kanye ne-lysine (i-lyr) kanye ne-lysine (i-lyr) kanye ne-lysine. Bahlanganisa ama-cetionic vesicles we-dodecyltrimylammonium bromide (DTAB) / LYS-Derivatives kanye / noma ama-ser- / amakhono asuselwa e-ecotoxicity kanye ne-hemolytic, ekhombisa ukuthi ukuhlanganiswa kwabo kwe-ecotoxicity kanye ne-vesicle-equkethe ama-aas abunobuthi obuncane kune-dtab evamile ye-DTAB.
URosa et al. Uphenya ngokubopha (Inhlangano) ye-DNA ukuze igxile kuma-amino acidi acidic Ngokungafani nama-cationicial desicactants ajwayelekile, abonakala evame ukuba yingozi, ukuxhumana kwe-cotionic amino acid surffactive kubonakala kungabi yingozi. I-cationic AAS isuselwe e-Arginne, esebenza ngokuzenzakalelayo ama-vesicles azinzile ngokuhlanganiswa nabasebenzi abathile be-anionic. I-Amino acid-based corosion-inhibitors nayo ibikiwe ukuthi ayinobuthi. Lezi zinto ezisetshenziswayo zithathwa kalula ngobumsulwa obuphezulu (kuze kufike ku-99%), izindleko eziphansi, kungathandeki kalula, futhi kuncibilike ngokuphelele kwimidiya enamanzi. Izifundo eziningana zikhombise ukuthi ama-sulfur - aqukethe ama-amino acid ama-surficis aphakeme ekuvinjelweni kwe-cororing.
Ocwaningweni lwakamuva, uPerinelli et al. Kubikwe iphrofayili egculisayo egculisayo yama-rhamnolipids ngokuqhathaniswa nokuqina okujwayelekile. Ama-rhamnolipipids ayaziwa ukuthi asebenza njengezikhunzi zemvume. Baphinde babike nomphumela wama-rhamnolipids ekubonisweni kwe-epithelial kwezidakamizwa ze-macromolecular.
08 Umsebenzi we-Antimicrobial
Umsebenzi we-antimicrobial we-surfactants ungahlolwa ngokuhlushwa okuphansi okuphezulu. Umsebenzi we-antimicrobial wezinto ezisetshenziswayo ezisuselwa e-Argine ufunde ngokuningiliziwe. Kutholakale amagciwane angemubi amaGram Umsebenzi we-antimicrobial we-surficroants uvame ukwanda ngokuba khona kwe-hydroxyl, i-cyclopropane noma izibopho ezingafakwanga ngaphakathi kwamaketanga e-acyl. Castillo et al. Kuboniswe ukuthi ubude bamaketanga we-Acyl kanye nenkokhiso emihle banquma inani le-HLB (i-Hydrophilic-Lipophilic Balance) ye-molecule, futhi lokhu kunomthelela emandleni abo okuphazamisa ulwelwesi. I-NEME-ACACLarginine Methyl Ester ingesinye isigaba esibalulekile se-cationic surfactants ngomsebenzi obanzi we-antimicrobial futhi iyinto ephansi futhi inobuthi obuphansi noma obunobuthi. Izifundo ngokuxhumana kwe-NEME-Accylarpunine Methyl-based Methyl-based Surfactants ene-1,2-propeltrioxyl-3-phosphorylcholine-3-phosphorylcholine-3-phosphorylcholine, ulwelwesi lwemodeli, kanye nezinto eziphilayo ezikhonjelwe ngaphakathi Lesi sigaba se-surfactants sine-antimicrobial enhle imiphumela ekhombisa ukuthi ama-surfactants anomsebenzi omuhle we-antibacterial.
Izakhiwo eziyi-09 ze-Rheological
Izakhiwo ezi-bherlogical ze-surfactants zidlala indima ebaluleke kakhulu ekunqumeni nasekubikezeleni izicelo zazo emikhakheni ehlukene, kubandakanya nokudla, amakhamishini, ukukhishwa kwamafutha, ukunakekelwa komuntu siqu kanye nemikhiqizo yokunakekelwa kwasekhaya. Kwenziwe izifundo eziningi ukudingida ubudlelwano phakathi kwe-viscoeliasticity ye-amino acid surfiactants ne-CMC.
Izicelo eziyi-10 embonini yezimonyo
Aas asetshenziswa ekwakhekeni kwemikhiqizo eminingi yokunakekelwa komuntu.I-Potassium N-Cocoyl Glyciate itholakala imnene esikhunjeni futhi isetshenziswa ekuhlanzeni kobuso ukuze isuse i-sludge ne-makeup. I-N-Acyl-L-Glutamic Acid inamaqembu amabili e-carboxyl, okwenza ukuthi kube namanzi amaningi. Phakathi kwalezi zi-aas, ama-aas asuselwa ku-C 12 fatty acids asetshenziswa kabanzi ekuhlanzeni kobuso ukuze asuse i-sludge ne-makeup. I-AAS ene-C 18 chain isetshenziswa njenge-emulsifiers emikhiqizweni yokunakekelwa kwesikhumba, kanye nosawoti we-n-lauryl alatine ayaziwa ukudala ama-fooms anokhilimu angacasuli esikhunjeni futhi angasetshenziswa ekwakhiweni kwemikhiqizo yokunakekelwa kwezingane. I-AAS esekelwe e-N-Laughl esetshenziswa ekuxubeni izinyo inokungcola okuhle okufana nensipho kanye nokusebenza okuqinile okuvinjelwe.
Emashumini ambalwa eminyaka adlule, ukukhethwa kwama-surficationnts for cosmetics, imikhiqizo yokunakekelwa komuntu kanye nemithi yezemithi igxile ekubikeni okuphansi, ubumnene, ubumnene ngokuthinta nokuphepha. Abasebenzisi bale mikhiqizo bayazi kakhulu ukucasuka okungaba khona, ubuthi kanye nezici zezemvelo.
Namuhla, aas asetshenziselwa ukwakha ama-shampoos amaningi, amadayi wezinwele kanye nensipho yokugeza ngenxa yezinzuzo zawo eziningi ngaphezulu komlingani wazo wendabuko kwezimonyo kanye nemikhiqizo yokunakekelwa komuntu.Ama-protein asuselwa kumaprotheni anezakhiwo ezifiselekayo ezidingekayo zemikhiqizo yokunakekelwa komuntu. Amanye ama-AA anamakhono okwakha amafilimu, kanti amanye anamakhono amahle okugcwala.
Ama-amino acid abalulekile ngokwemvelo avela ezinyaweni ezinhle kakhulu ku-stratum Corneum. Lapho amaseli e-Epidermal efa, abe yingxenye ye-stratum Corneum kanye namaprotheni e-intracellure kancane kancane adilizwa ama-amino acid. Lawa ma-amino acid athunyelwa ngaphezulu emgqeni we-stratum, lapho amunca izinto ezinamafutha noma ezinamafutha ku-epidermal stratum corneum, ngaleyo ndlela enze ngcono ukuqina kwesikhumba. Cishe i-50% ye-Natural Moisturizing factor esikhunjeni yakhiwa ama-amino acid kanye ne-pyrloridone.
I-Collagen, isithako esijwayelekile sohlanga, futhi iqukethe ama-amino acid agcina isikhumba sithambile.Izinkinga zesikhumba ezinjengobulukhuni nokubumbana zibangelwa engxenyeni enkulu nokuntuleka kwama-amino acid. Olunye ucwaningo lubonise ukuthi ukuxuba i-amino acid ngogcoba okugcotshwe isikhumba, kanti izindawo ezithintekile zibuyele esimweni sazo esijwayelekile ngaphandle kokuba izibazi ze-keloid.
Ama-amino acid nawo atholakale ukuthi awusizo kakhulu ekunakekeleni ama-cutics alimazayo.Izinwele ezomile, ezingenasiphelo zingakhombisa ukwehla kwama-amino acid e-amine e-corneum elonakalisiwe kakhulu. Ama-amino acid anekhono lokungena e-cuticle kwi-haft yezinwele bese edonsa umswakama esikhunjeni.Leli khono elisezingeni le-amino acid lisenza ukuthi lilusizo kakhulu kuma-shampoos, amadayi ezinwele, amathafa wezinwele, ama-hair conditioners, kanye nokuba khona kwama-amino acid kwenza izinwele ziqine.
Izicelo eziyi-11 kwizimonyo zansuku zonke
Njengamanje, kunesidingo esikhulayo sezakhi zokuhlanza ezisuselwa ku-amino acid emhlabeni wonke.I-AAS yaziwa ukuthi inekhono elingcono lokuhlanza, ikhono lokuhlanza kanye nendwangu ethambisa izakhiwo, ezibenza zilungele okokuhlanza kwasekhaya, ama-shampoos, ukugeza umzimba kanye nezinye izinhlelo zokusebenza.I-Amphoteric Acid Acid Amphoteric AAS kubikwa ukuthi ingukuhlanzeka okusebenzayo kakhulu nge-Prelating Properties. Ukusetshenziswa kwezithako zokuhlanza okubandakanya ama-N-ALKYL-β-Aminoethoxy acid kutholakale kunciphisa ukucasuka kwesikhumba. Ukwehla kwamanzi okuhlanza okuqukethe i-n-cocoyl-β-aninopropionate kubikwe ukuthi kungukuhlanza okusebenzayo kwamabala kawoyela ezinhlamvini zensimbi. I-aninoCarboxyylic acid eqinile, c 14 chohch 2 NHCH 2 Cooona, nayo ikhonjiswe ukuthi inokuhlanza okungcono futhi isetshenziselwa ukuhlanza i-acingiles, amakhaphethi, i-acitopropropionaition, njll.
Ukulungiswa kwezinto zokuhlanza ezisuselwa ku-n- (n'-chain acyl-β-alanine kubikwe nguKeigo noTatsuya kuKatsuya ku-patent yabo yokugeza namandla okugeza nokuqina. UKao wahlakulela ukwakhiwa kwe-detergent ngokususelwa ku-n-acyl-1 -N-HYDROXY-β-Alanine futhi wabika ukucasuka kwesikhumba okuphansi, ukumelana kwamanzi aphezulu kanye namandla aphezulu okususa amabala.
Inkampani yaseJapan Ajinomoto isebenzisa ama-Ajinomoto anobuthi anobuthi futhi ancipha kalula ku-L-Glutamic acid, i-L-Arginine kanye ne-L-Lysine njengezithako eziyinhloko kuma-shampoos, ama-detegents kanye nezimonyo (Umdwebo 13). Amandla we-enzyme angezelelwa ekwakhiweni kwama-detergent ukususa ama-protein fouling nawo kubikwe. I-N-Acyl AAs ethathwe ku-glutamic acid, i-alanine, i-methylllycine, i-serine kanye ne-aspline acid sekubikwe ukusetshenziswa kwazo njengowokuhlanza okungamanzi okuhle kwezixazululo zamanzi. Lezi zinto ezisetshenziswayo azikhulisi ukubonakala nhlobo, ngisho nasemazingeni okushisa aphansi kakhulu, futhi zingashintshwa kalula kusuka kumkhumbi wesitoreji wedivayisi ye-Foaming ukuthola amagwebu angenazinhlamvu.
Isikhathi sePosi: Jun-09-2022

