

I-Shrinkage Force yanoma yibuphi ubude beyunithi ebusweni boketshezi ibizwa ngokuthi ukungezwani komhlaba, futhi iyunithi yi-N. · M-1.

Impahla yokwehlisa ukungezwani kwe-solvent ibizwa ngokuthi yi-surce finct, futhi into enale mpahla ibizwa ngokuthi yinto esebenzayo.
Into esebenzayo engaphezulu engabopha ama-molekyuli esikhaleni samanzi namafomu namanye ama-miclelles kanye nezinye izinhlangano, futhi inomphumela wokumanzisa, ngenkathi inomphumela wokumanzisa, ngenkathi inethonya lokugeza, njll. Ibizwa ngokuthi i-Surfactant.
Surfactant is organic compounds with special structure and property, which can significantly change the interfacial tension between two phases or the surface tension of liquids (generally water), with wetting, foaming, emulsifying, washing and other properties.
Ngokwesakhiwo, ama-surfactants anesici esijwayelekile ngoba aqukethe amaqembu amabili emvelo ehlukile kuma-molecule abo. Esikhathini esisodwa uchungechunge olude lweqembu elingewona ama-polar, luncibilike emafutheni naku-inserfoble emanzini, elaziwa nangokuthi neqembu le-hydrophobic noma iqembu eliphindayo lamanzi. Iqembu elinjalo lamanzi livame ukufana namaketanga amade ama-hydrocarbons, kwesinye isikhathi futhi ne-organic fluorine, i-silicon, i-organicoosphate, i-organin chain, njll. Ngakolunye uhlangothi i-Water-Suplel Group, iqembu le-Hydrophilic noma iqembu eliphendulayo kawoyela. Iqembu le-hydrophilic kumele libe ne-hydrophilic eyanele ukuqinisekisa ukuthi wonke ama-surfactants ancibilika emanzini futhi ane-solubity edingekayo. Njengoba ama-surfactants aqukethe amaqembu e-hydrophilic ne-hydrophobic, angancibilika okungenani esigabeni se-liquid. Le mpahla ye-hydrophilic ne-lipophilic ye-surdophantant ibizwa ngokuthi yi-Amphilicicity.


I-Surfactant uhlobo lwama-molecule we-aphphiphilic anamaqembu e-hydrophobic nama-hydrophilic. Amaqembu we-Hydrophobic asetshenziswa ngokuvamile akhiwa ngama-hydrocarbons amade, anjenge-ALKYL C8 ~ C20, i-Alkylphenyl (Alkyl Carbon Tom, inombolo ye-Alkylphenys (i-Alkyl Carbon Tom ingu-8 ~ 16) nokunye. Umehluko omncane phakathi kwamaqembu e-hydrophobic ikakhulukazi ezinguquko ezihlelekile zamaketanga we-hydrocarbon. Futhi izinhlobo zamaqembu e-hydrophilic ziningi, ngakho-ke izakhiwo ze-surffactants zihlobene kakhulu namaqembu we-hydrophilic ngaphezu kosayizi kanye nokwakheka kwamaqembu e-hydrophobic. Ushintsho oluhlelekile lwamaqembu ama-hydrophilic lukhulu kunalawo amaqembu ama-hydrophobic, ngakho-ke ukuhlukaniswa kwama-surfactants ngokuvamile kususelwa esakhiweni samaqembu ama-hydrophilic. Lokhu kuhlukaniswa kususelwa ekutheni iqembu le-hydrophilic liyi-ionic noma cha, futhi lihlukaniswe nge-anionic, i-cationic, i-onion, zwitterionionic kanye nezinye izinhlobo ezikhethekile ze-surficactants.

① Ama-adsorption ama-surfactants e-interfac
Ama-molecule asetshenzisiwe ama-molecule we-amphiphilic anamaqembu e-lipophilic namaqembu e-hydrophilic. Lapho i-survitant ichithwa emanzini, iqembu layo le-hydrophilic likhangwa emanzini, kanti iqembu laso le-lipophilic lixoshwa ngamanzi ancipha izigaba ezimbili, okuholela ekuxhumekweni kwezigaba ezimbili, okunciphisa ukungezwani kwezigaba ezimbili phakathi kwezigaba ezimbili. Ama-molecules amaningi asetshenziswayo (noma i-ion) akhishwe ku-interface, kuncishiswa okukhulu ekunciphiseni ukungezwani.
② Ezinye izakhiwo zolwelwesi lwe-adsorption
Ingcindezi engaphezulu yolwelwesi lwe-adsorption: I-Surfactant Adsorption esibonakalayo segesi-liquid ukwenza ulwelwesi lwe-adsorption, ishidi elisentabeni ligxilisa ulwelwesi olucasulayo, futhi ulwelwesi lukhiqiza ingcindezi eshidini elintantayo, elibizwa ngokuthi ingcindezi ephepheni elintantayo, elibizwa ngokuthi ingcindezi eshidini elintantayo, elibizwa ngokuthi ingcindezi eshidini elintantayo, elibizwa ngokuthi ingcindezi eshidini elintantayo, elibizwa ngokuthi ingcindezi eshidini elintantayo, elibizwa ngokuthi ingcindezi eshidini elintantayo, elibizwa ngokuthi ingcindezi eshidini elintantayo
I-Supcusity Engaphezulu: Njengomfutho we-Surface, i-Viscosity Engaphezulu iyimpahla eboniswa ulwelwesi lwama-molecular olungaphansi. Kumiswe ngendandatho ye-petal yensimbi yensimbi enhle, ukuze iplanethi yayo ixhumana ne-Plane lamanzi, ijikeleze indandatho yeplatinamu, indandatho yeplatinamu ngokubonakala kwezithiyo zamanzi, ngakho-ke ukubola kancane kancane, ngokusho kokubonakala komhlaba kungalinganiswa. Indlela yile: Okokuqala, ukuhlolwa kwenziwa endaweni ehlanzekile yamanzi ukukala ukubola kwe-amplitude, bese kuba khona ukubola ngemuva kokuhlelwa kolwelwesi lwaphezulu kulinganiswa, futhi ukubonwa kolwelwesi olungaphezulu kuthathwe umehluko phakathi kwalokhu okubili.
Ukubonakala komhlaba kuhlobene kakhulu nobuningi be-membrane engaphezulu, futhi njengoba ulwelwesi lwe-adsorption lunengcindezi engaphezulu nokubonakala, kumele kube nokuqina. Ukucindezela kwengcindezi engaphezulu kanye nokuphakama kwe-viscosity ye-adsorbed membrane, kuphakama i-modulus yayo ye-elastic. I-modulus e-Elestic yoMfutho we-Adsorption wangaphandle ibalulekile kwinqubo yokuzinza kwe-bubble.
③ ukwakheka kweMicelles
Izixazululo ze-Dilictete of Surfactants Lalela imithetho elandelwa izixazululo ezifanele. Inani le-adsorbed elisezingeni eliphakeme ebusweni bekhambi likhuphuka ngokuqoqwa kwesixazululo, futhi lapho okuhlushwa kufinyelela noma kudlula inani elithile, futhi le maloluyuseli asetshenzisiwe ayisakhuphuke, futhi la ma-molecule asetshenziswayo asetshenziswayo asakhuphuka ngendlela ye-haphazard noma ngendlela ethile. Zombili ukuzilolonga nothiyori zibonisa ukuthi ziveza izinhlangano ezisetshenziswayo, futhi lezi zinhlangano zibizwa ngeMicelles.
Ukuhlushwa kwe-micelle okubucayi (CMC)
④ Amanani we-CMC ama-surffactants ajwayelekile.

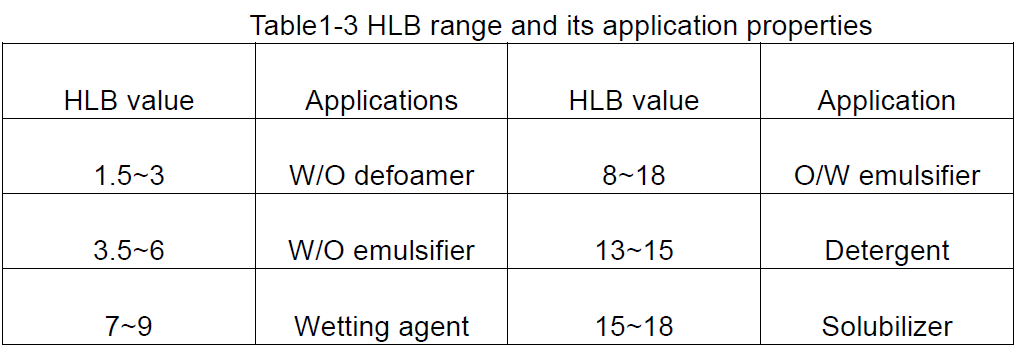
I-HLB yisifinyezo se-hydrophilelialialialie yebhalansi, ekhombisa ibhalansi ye-hydrophilic ne-lipophilic yamaqembu ama-hydrophilic nama-lipophilic we-surdrophilic kanye ne-lipophilic ye-surdicant, okungukuthi, inani le-HLB le-surviant. Inani elikhulu le-HLB libonisa i-molecule ene-hydrophicility eqinile kanye ne-lipophilicity ebuthakathaka; Ngakolunye uhlangothi, i-lipophilicity eqinile ne-hydrophilicity ebuthakathaka.
① Izinhlinzeko zenani le-HLB
Inani le-HLB inani elilinganayo, ngakho-ke lapho inani le-HLB lithuthukiswa, njengenani le-HLB le-Pardfinic, elinamanani ama-sodium dodecyl, elinamanani angama-40 ngokuvamile ngaphakathi kwe-1 kuye ku-40. Ngokuvamile, ama-emulsifiers Amanani we-HLB angaphansi kwe-10 angama-lipophilic, kuyilapho ayedlula kune-10 yi-hydrophilic. Ngakho-ke, ukuphela okuguqukayo kusuka ku-lipophilic ku-hydrophilic cishe kungu-10.
Kususelwa kumanani we-HLB ama-surficasternts, umbono ojwayelekile wokusetshenziswa kwawo okungenzeka ungatholwa, njengoba kukhonjisiwe kuThebula 1-3.

Ama-liquitsheli amabili angenakuqhathaniswa, ahlakazeka kwelinye njengezinhlayiya (amaconsi noma amakristalu aloketshezi) akha uhlelo olubizwa ngokuthi yi-emulsion. Lolu hlelo lungazinzile ngokungazinzi ngenxa yokwanda endaweni yomngcele yotshwala obubili lapho kudalwa khona i-emulsion. Ukuze wenze i-emulsion izinzile, kuyadingeka ukwengeza ingxenye yesithathu - i-emulsifier ukuze unciphise amandla okuzijabulisa wohlelo. I-Emulsifier ingeyama-survifier, umsebenzi wayo oyinhloko ukudlala indima ye-emulsion. Isigaba se-emulsion esikhona njengamaconsi sibizwa ngokuthi isigaba esihlakazekile (noma isigaba sangaphakathi, isigaba esincishisiwe), futhi esinye isigaba esihlangene sibizwa ngokuthi yi-Dispivering Medium (noma isigaba esingaphandle).
① Emulsifiers kanye ama-emulsions
Ama-emulsion ajwayelekile, isigaba esisodwa siyisisombululo samanzi noma samanzi, esinye isigaba yizinto eziphilayo ezingabonakali ngamanzi, amafutha ahlukaniswe ngamafutha, ahlakazeke emanzi azokwakha amafutha akhona, ahlakazeke ngamafutha azokwakha i-emulsion yohlobo lwamafutha, avezwe njenge-W / O (amanzi / oyela). Uhlobo oluyinkimbinkimbi lwamanzi-in-water-in-wamanzi
Ama-emulsifiers asetshenziselwa ukuqinisa ama-emulsions ngokunciphisa ukungezwani kwezingcweti nokwakha ulwelwesi lokuhlangana kwe-molecule olulodwa.
E-Emulsation yezidingo ze-emulsifier:
A: I-Emulsifier kumele ikwazi ukucebisa noma ukucebisa ukuxhumana phakathi kwezigaba ezimbili, ukuze ukungezwani kwezinye izinyembezi kuncishisiwe;
B: I-emulsifier kumele inikeze izinhlayiya eziya ekushajaneni, ukuze ukuthuthukiswa kwe-elektrosts phakathi kwezinhlayiya, noma kwakha ulwelwesi oluzinzile, oluvikela kakhulu oluvikeleke ezizungeze izinhlayiya.
Ngakho-ke, into esetshenziswa njenge-emulsifier kumele ibe namaqembu we-aphphiphilic ukuze ahluze, futhi ama-surfactants angahlangabezana nale mfuneko.
Izindlela zokulungiselela ama-emulsion kanye nezinto ezithinta ukuqina kwama-emulsions
Kunezindlela ezimbili zokupheka ama-emulsion: umuntu ukusebenzisa indlela yemishini yokuhlakaza uketshezi ezinhlayiyeni ezincane kolunye uketshezi, olusetshenziswa kakhulu embonini ukulungiselela ama-emulsions; Okunye ukuncibilikisa uketshezi esimweni samangqamuzana kolunye uketshezi, bese kukwenza ukuthi kuhlangane kahle ukuze kwakhiwe ama-emulsions.
Ukuqina kwe-emulsion yikhono le-anti-particle aggregation eliholela ekuhlukaniseni isigaba. Ama-emulsions amasistimu angazinzile anamandla amakhulu wamahhala. Ngakho-ke, okuthiwa ukuqina kwe-emulsion empeleni kuyisikhathi esidingekayo sohlelo ukufinyelela ukulingana, okungukuthi, isikhathi esidingekayo sokuhlukaniswa kolunye koketshezi ohlelweni oluzokwenzeka.
Lapho ulwelwesi lokuzijabulisa olunolaka olunamafutha, ama-acid amafutha kanye namafutha amine namanye ama-molecule e-polar, ama-membrane arganic ephakeme kakhulu. Lokhu kungenxa yokuthi, ku-serfacial adsorption ungqimba wama-molecule e-emulsifier kanye notshwala, ama-acid kanye nama-amoreces namanye ama-polar molecule ukwakha "okuyinkimbinkimbi, ukuze amandla okulemelana nawo akhuphuke.
Ama-Emulsifiers aqukethe okungaphezulu kwama-surfactants abizwa ngama-emulsifiers ahlanganisiwe. I-empulsifier exubile e-adsorbed esibonakalayo samanzi / samafutha; Isenzo se-intermolecular singakha izakhiwo. Ngenxa yesenzo se-intermolecular esinamandla, ukungezwani kwe-interfacial kuncishiswa kakhulu, inani le-emulsifier adsorbed endaweni ebonakalayo lenyuka kakhulu, ukwakheka kwe-membrane density inyuswa, amandla ayanda.
Imali ekhokhwa ubuhlalu owuketshezi inomthelela omkhulu ekuqineni kwe-emulsion. Ama-Emulsions azinzile, ubuhlalu bawo owuketshezi ngokuvamile ngokuvamile bathweswa icala. Lapho kusetshenziswa i-ionic emulsifier, i-emulsifier ion adsorbed e-interface ineqembu layo le-lipophilic lifakwe esigabeni samafutha neqembu le-hydrophilic lisesigabeni samanzi, ngaleyo ndlela senze ubuhlalu obuwuketshezi. Njengoba i-emulsion ubuhlalu ngecala elifanayo, baphindiselana, hhayi lula ukucasuka, ukuze ukuqina kuyanda. Kungabonakala ukuthi ama-emulsifier ion adderbed on the beads, kukhule kakhulu, kukhulisa ubuhlalu kusuka ku-agglomeration, kusiza kakhulu uhlelo lwe-emulsion.
I-viscosity ye-Emulsion Displion Medid inethonya elithile ekuqineni kwe-emulsion. Ngokuvamile, ukukhuphuka kwendlela yokuhlakazeka okuphakathi nendawo, kukhuphuka ukuqina kwe-emulsion. Lokhu kungenxa yokuthi i-viscosity ephakathi nendawo ephakathi, enomthelela oqinile ekusondelweni kwe-brownia ebuhlalu uketshezi futhi ibambezele phansi ukushayisana phakathi kobuhlalu obuwuketshezi, ukuze uhlelo lube luzinzile. Imvamisa, izinto ze-polymer ezingachithwa kuma-emulsion zingakhuphula ukubonwa kohlelo futhi zenze ukuqina kwama-emulsions aphezulu. Ngaphezu kwalokho, ama-polymers nawo angakha ulwelwesi oluqinile lokuzijabulisa, okwenza uhlelo lwe-emulsion luzinze ngokwengeziwe.
Kwezinye izimo, ukungezwa kwe-powder eqinile kungenza futhi i-emulsion ivame ukuzinza. I-Solid Powder isemanzini, uwoyela noma isikhombimsebenzisi, kuya ngamafutha, emanzini emandleni okumanzi we-powder eqinile, uma i-powder eqinile ingagcinwanga ngokuphelele ngamanzi, kepha i-wailef i-oyela kanye nesibonisi samafutha.
I-powder eqinile ayenzi i-emulsion izinzile ngoba i-powder iqoqwe e-interface ikhulisa ulwelwesi lwe-interfacial, olufana ne-adposic e-adsorption yama-molecule e-emulsifier, ngakho-ke kugxilwe kakhulu i-apulalsion.
Ama-surfactants anamandla okukhulisa kakhulu insourbity ye-insoluble noma ngaphansi kwamanzi ancibilikayo ancibilika ama-organic ngemuva kokukhipha izimoto esikhaleni esinamanzi, futhi isixazululo sisobala ngalesi sikhathi. Lo mphumela we-micelle ubizwa ngokuthi yi-solubilization. I-Surfactant engakhiqiza i-tubilization ibizwa ngokuthi yi-solubililizer, kanye nento e-organic ebekwe yi-solubilized ibizwa ngokuthi yi-pulibilized ndaba.

UFoam udlala indima ebalulekile enqubweni yokuwasha. I-Foam uhlelo lokuhlakazeka lapho igesi ihlakazwa ngoketshezi noma iqinile, igesi njengesigaba esihlakazekile kanye noketshezi olubizwa ngokuthi i-foam eluhlaza, ngenkathi okusabizwa ngokuthi ipulasitiki eluhlaza, elibizwa nge-fopula elibi, ligingy ingilazi, ingilazi ye-fold, ingilazi ye-fold, ingilazi ye-fold, ingilazi ye-fold, i-pooment cement verc
(1) ukwakheka kwe-foam
Nge-Foam sisho lapha i-aggregate yama-bubble amoya ahlukaniswe ngolwelwenzi oluwuketshezi. Lolu hlobo lwe-bubble luhlala lukhuphuka ngokushesha endaweni ewuketshezi ngenxa yomehluko omkhulu ekubunjweni phakathi kwesigaba esihlakazekile (igesi) kanye nendlela yokuhlakazeka (ehlanganiswe ne-viscosity ephansi yoketshezi.
Inqubo yokwenza i-bubble ukuletha inani elikhulu legesi ku-ketshezi, futhi ama-bubble ku-liquid asheshe abuyele ebusweni, akha i-aggregate yama-bubble ahlukaniswe yimali encane ehlukaniswe yimali encane.
I-Foam inezimpawu ezimbili ezibalulekile ngokuya nge-morphology: munye ukuthi ama-bubble njengesigaba esihlakazekile kwesinye isikhathi ama-polyhedral, lapho kuthambekele kwefilimu kube yi-polyhedral, lapho amafilimu awuketshezi afinyelela ku-bubble; Okwesibili ukuthi uketshezi olumsulwa alukwazi ukwakha igwebu elizinzile, uketshezi olungakha i-foam okungenani okungenani ezimbili noma ngaphezulu. Izisombululo ezinamanzi ezifuywayo zijwayelekile ezinhlelweni ezithambekele ku-foam Generation, kanye nekhono labo lokukhiqiza ama-foam lihlobene nezinye izakhiwo.
Ama-surfactants anamandla amahle amagwebu abizwa ngokuthi ama-foaming agents. Yize i-ejenti ye-Foaming inekhono elihle legwebu, kepha igwebu elakhiwe kungenzeka lingakwazi ukugcina isikhathi eside, okungukuthi, ukuqina kwalo akukuhle. Ukuze ulondoloze ukuqina kwegwebu, imvamisa ku-ejenti ye-foaming ukwengeza izinto ezingakhulisa ukuqina kwegwebu, into ebizwa ngokuthi i-foury stabilizer, i-stabilizer esetshenziswayo yi-lauryl diethanolamine ne-dodecyl dimethylamine noDodecyl Dimethylalalamine noDodecyl Dimethylalalamine noDodecyl Dimethyyamine oxide.
(2) Ukuqina kwegwebu
I-Foam uhlelo olungazinzile ngokwezifiso kanye nomkhuba wokugcina ukuthi indawo ephelele engaphezulu yoketshezi ngaphakathi kohlelo iyancipha ngemuva kokuthi i-bubble iphuliwe futhi amandla wamahhala ayancipha futhi amandla wamahhala ayancipha. Inqubo yokudonswa kwenqubo yinqubo lapho ulwelwesi oluwuketshezi oluhlukanisa igesi likhulu futhi linciphile kuze kube yilapho liphuka. Ngakho-ke, izinga lokuqina kwegwebu linqunywa ikakhulu ngejubane lokukhishwa koketshezi namandla wefilimu eliwuketshezi. Izici ezilandelayo zinomthelela nalokhu.
(3) Ukubhujiswa kwe-Foame
Isimiso esiyisisekelo sokubhujiswa kwe-Foam ukushintsha izimo ezikhiqiza igwebu noma ukuqeda izinto eziqinile zegwebu, ngakho-ke kunezindlela zombili nezamakhemikhali zokufakelwa.
Ukungcolisa ngokomzimba kusho ukuguqula imibandela yokukhiqizwa kwe-foam ngenkathi kugcinwa ukwakheka kwamakhemikhali kwesisombululo segwebu, njengokuphazamiseka kwangaphandle, izinguquko zokushisa noma ukwelashwa kwe-ultrasonic zonke izindlela ezisebenza kahle zokuqeda igwebu.
Indlela ye-Chemical Devoaming ukwengeza izinto ezithile zokusebenzisana ne-ejenti ye-Foaming ukunciphisa amandla wefilimu eliwuketshezi futhi ngaleyo ndlela kunciphise ukuqina kwegwebu ukufeza inhloso yokungcolisa, izinto ezinjalo zibizwa ngokuthi ama-desoamer. Iningi lama-desoamers lingama-surfactants. Ngakho-ke, ngokusho komshini wokuphambanisa, i-defouamer kufanele ibe nekhono elinamandla lokunciphisa ukungezwani komhlaba, okulula ku-adcerb ebusweni, kanye nokuxhumana phakathi kwama-molecule akwa-adsorption abuthakathaka, ama-molecule e-adsorption ahlelwe ngesakhiwo esinyuluki xaxa.
Kunezinhlobo ezahlukahlukene ze-desoamer, kepha ngokuyisisekelo, bonke bayizisebenzi ezingekho emthethweni. Ama-sursuctants angewona ama-ionic anezakhiwo ezilwa namagwebu eduze noma ngaphezulu kwephuzu lakhe lefu futhi zivame ukusetshenziswa njenge-desoamers. Ama-alcohols, ikakhulukazi ama-alcohols anesakhiwo segatsha, ama-acids aciji namafutha acidia, ama-polyamides, ama-phosphate esters, uwoyela we-silicone, njll. Zivame ukusetshenziswa ama-desoumers amahle kakhulu.
(4) Foam nokugeza
Akukho ukuxhumana okuqondile phakathi kwegwebu nokugeza ukusebenza kanye nenani le-foam alikhombisi ukusebenza kokugeza. Isibonelo, ama-surfactants angezona angenayo anezindawo ezimbalwa ezinamafutha kunezinsipho, kepha ukuwohloka kwawo kungcono kakhulu kunezinsipho.
Kwezinye izimo, igwebu lingasiza ekususeni ukungcola noGrime. Isibonelo, lapho ugeza izitsha ekhaya, igwebu lesihlanzi lithatha amaconsi kawoyela nalapho eklama amakhaphethi, i-foam isiza ukuthatha uthuli, i-powder nokunye ukungcola okuqinile. Ngaphezu kwalokho, amagwebu ngezinye izikhathi azosetshenziswa njengenkomba yokusebenza kokuhlanza. Ngoba uwoyela onamafutha unomphumela ovimbayo ku-foam wesiteleka, lapho kukhona uwoyela omningi kakhulu futhi ukhipha kancane kakhulu, akukho foam okuzokhiqizwa noma igwebu lasekuqaleni lizonyamalala. I-Foam nayo kwesinye isikhathi ingasetshenziswa njengenkomba yokuhlanzeka kwesisulu, njengoba inani le-foam kukhambi lokuhlanza livame ukwehla ngokuncishiswa kokuhlanza, ngakho-ke inani le-foam lingasetshenziswa ukuhlola izinga lokuvutha.
Ngomqondo obanzi, ukugeza yinqubo yokususa izakhi ezingafuneki entweni ezogezwa futhi ifinyelele kwinjongo ethile. Ukugeza ngomqondo ojwayelekile kubhekisele kwinqubo yokususa ukungcola ebusweni bomthwali. Ekugezekeni, ukuxhumana phakathi kokungcola kanye nomthwali kubuthakathaka noma kuqedwe ngesenzo sezinto ezithile zamakhemikhali (isib. Njengoba izinto okufanele zigezwe futhi ukungcola kuzosuswa ziyehluka, ukugeza kuyinqubo eyinkimbinkimbi kakhulu futhi inqubo eyisisekelo yokuwasha ingavezwa ebudlelwaneni obulula.
Carrie · Ukuxoshwa dirt + Dricker = Carrier + Umbala · Dyergent
Inqubo yokugeza ngokuvamile ingahlukaniswa izigaba ezimbili: Okokuqala, ngaphansi kwesenzo sokuhlanza, ukungcola kuhlukaniswe nomthwali wayo; Okwesibili, ukungcola okuvinjelwe kuhlakazeka futhi kumiswe endaweni ephakathi nendawo. Inqubo yokuwasha inqubo eguquguqukayo kanye nokungcola okuhlakazeka futhi kumiswe endaweni ephakathi nendawo kungahle kuphinde kwaqedwa kusuka enkabeni kuya entweni egezwa. Ngakho-ke, okokuhlanza okuhle kufanele kube nekhono lokuhlakaza futhi umise ukungcola futhi kuvikele ukungcola kokungcola, ngaphezu kwekhono lokususa ukungcola kumthwali.
(1) izinhlobo zokungcola
Noma entweni efanayo, uhlobo, ukwakheka kanye nenani lokungcola kungahluka ngokuya ngemvelo lapho isetshenziswa khona. Ukungcola komzimba ongamafutha ikakhulukazi amafutha ezilwane namamifino (njengamafutha anamaminerali, amafutha angcolile, ukungcola kwamalahle, njll. Ngokwezithako zomzimba womuntu, njll. Ngokwesithukuthezi, ukungcola kusuka ekudleni, njengamabala ezithelo, ukupheka amabala kawoyela, amabala athile, isitashi, njll.; Ukungcola kusuka kwizimonyo, njenge-lipstick, i-nail Polish, njll; ukungcola kusuka emkhathini, njengokuhlanza, uthuli, udaka, njll; Abanye, njenge-inki, itiye, ukuhlanganisa, njll. Iza ngezinhlobo ezahlukahlukene.
Izinhlobo ezahlukahlukene zokungcola zivame ukuhlukaniswa izigaba ezintathu eziphambili: ukungcola okuqinile, ukungcola okuwuketshezi kanye nokungcola okukhethekile.
① ukungcola okuqinile
Ukungcola okuqinile okuvamile kufaka izinhlayiya zomlotha, udaka, umhlaba, ukugqwala kanye nekhabhoni emnyama. Iningi lalezi zinhlayiya linecala elikagesi ebusweni bawo, iningi labo likhokhiswa kabi futhi lingafakwa kalula ezintweni ze-fiber. Ukungcola okuqinile kuvame ukuncipha emanzini, kepha kungahlakazwa futhi kumiswe ngezixazululo zokuhlanza. Ukungcola okuqinile ngephuzu elincane elincanyana kunzima ukuwasusa.
② ukungcola okuwuketshezi
Ukungcola okuwuketshezi kuncibilika kakhulu uwoyela, kufaka phakathi amafutha ezitshalo namafutha, ama-fatty acid, amafutha, uwoyela wamafutha, amafutha anamafutha kanye noxides babo. Phakathi kwazo, amafutha ezitshalo kanye nezilwane, ama-acid ama-acid kanye nama-alkali angenzeka, ngenkathi amafutha anamafutha, amafutha anamafutha awathathwa ngotshwala, kepha angancibilika ngotshwala, futhi angancibilika ama-organic arganication, kanye nokuhlakazwa kwezixazululo zamanzi. Ukungcola okunoshukela ongenawo-kawoyela ngokuvamile kunobutho obuqinile ngezinto ze-fiber, futhi kufakwe kahle ama-adderbed on Fibers.
③ ukungcola okukhethekile
Ukungcola okukhethekile kufaka amaprotheni, isitashi, igazi, izifutho zomuntu ezinjengokujuluka, iSebum, umchamo kanye nejusi yezithelo nejusi letiye. Iningi lalolu hlobo lokungcola lungahlelwa ngamakhemikhali futhi luhlelwe ngokuqinile ezintweni ze-fiber. Ngakho-ke, kunzima ukugeza.
Izinhlobo ezahlukahlukene zokungcola azivamisile ukutholakala zodwa, kepha zivame ukuxutshwa ndawonye futhi zifakwe e-added entweni. Ukungcola kwesinye isikhathi kungaba yi-onidized, kubolile noma kubonwe ngaphansi kwamathonya angaphandle, ngaleyo ndlela kwakha ukungcola okusha.
(2) Ukunamathela kokungcola
Izingubo, izandla njll zinganakwa ngoba kukhona uhlobo oluthile lokuxhumana phakathi kwento kanye nokungcola. Ubhuti unamathela ezintweni ngezindlela ezahlukahlukene, kepha azikho ngaphezu kokunamathela ngokomzimba nangamakhemikhali.
①ukunamathela kosofu, uthuli, udaka, isihlabathi namalahle ezingutsheni kungukunamathela ngokomzimba. Ngokuvamile, ngokusebenzisa lokhu kunamathela kokungcola, futhi indima ephakathi kwento enamabala ibuthakathaka, ukususwa kokungcola nakho kulula. Ngokusho kwamabutho ahlukene, ukunamathela ngokomzimba kokungcola kungahlukaniswa kube nokunamathelisa ngomshini kanye nokunamathela ngogesi.
A: Ukunamathelisa okwenziwe ngomshini
Lolu hlobo lokunamathela ikakhulukazi lubhekisela ekunamathisweni kokungcola okuthile okuqinile (isib., Uthuli, udaka nesihlabathi). Ukunamathela kwemishini kungenye yezinhlobo ezibuthakathaka zokunamathela kokungcola futhi kungasuswa cishe ngezindlela ezenziwa ngomshini, kepha lapho ukungcola kuncane (<0.1um), kunzima ukuwasusa.
B: Ukunamathela kwe-electrostatic
Ukunamathela kwe-elektrostutic kubonakaliswa ikakhulukazi ekwenzeni izinhlayiya ezingcolile ezikhokhiswayo ezintweni eziphikisayo ezikhokhiswayo. Izinto eziningi ze-fibrous zikhokhiswa kabi emanzini futhi zingalandelwa kalula ukungcola okukhokhiswa kahle, njenge-lime. Okunye ukungcola, yize kuthelwe kabi, njengezinhlayiya ezimnyama zekhabhoni ezixazululweni zamanzi, kungalandela imicu ngamabhuloho e-Ionic (i-ion phakathi kwabo ebhulobheni eliphikisayo) okwakhiwa ngama-ion ama-study emanzini (isib.
Isenzo se-elekthrostiki sinamandla kunesenzo esilula semishini, okwenza ukususwa okungcolile kunzima.
② Adhesion Chemical
Ukunamathela kwamakhemikhali kubhekisele entombini yokungcola esebenza entweni ngamakhemikhali wamakhemikhali noma we-hydrogen. Isibonelo, ukungcola okuqinile kwe-polar, amaprotheni, ukugqwala nokunye ukunamathela ezintweni ze-fiber, imicu iqukethe ama-carboxyl, amaqembu namanye amathayi, amaqembu namafutha angcolile alula ukwakha ama-hydrogen bond. Amabutho amakhemikhali ngokuvamile aqinile futhi ukungcola ngakho-ke kubunyezwe ngokuqinile entweni. Lolu hlobo lokungcola lunzima ukulususa ngezindlela ezijwayelekile futhi ludinga izindlela ezikhethekile zokubhekana nazo.
Izinga lokunamathela kokungcola lihlobene nohlobo lokungcola ngokwalo kanye nohlobo lwento elandelwa kulo. Ngokuvamile, izinhlayiya zinamathela kalula ezintweni ezifiphele. Uma kuncane ukuthungwa kongcolile okuqinile, okunamandla okunamathela. Ukungcola kwe-Polar ezintweni ze-Hydrophilic ezifana nokotini kanye nengilazi kunamathela kakhulu kunodoti olungelona olungelonamba. Ukungcola okungewona ama-polar kunamathela kakhulu kunodoti olunamakhaza, njengamafutha e-polar, uthuli nodaka, futhi kulula ukulususa futhi kuhlanzeke.
(3) Mechanism yokususa imfucuza
Inhloso yokuwasha ukususa ukungcola. Endaweni ephakathi nendawo yokushisa (ikakhulukazi amanzi). Kusetshenziswa imiphumela ehlukahlukene yomzimba neyomakhemikhali yokuhlanza noma ukuqeda umphumela wokungcola kanye nezinto ezihlanjiwe, ngaphansi kwesenzo samandla athile emishini (njengokugula kwezandla, umthelela wamanzi), ukuze kuncishiswe izinto ezingcolile ejele.
①shini ① yokususwa okungcolile okuwuketshezi
A: emanzi
Ukuncenga okuwuketshezi kusekelwe kakhulu ngowoyela. Ama-oyili anamabala ezinto ezinamanzi kakhulu futhi asakaze okuningi noma ngaphansi njengefilimu kawoyela ebusweni bezinto ezingcolile. Isinyathelo sokuqala esenzweni sokuwasha ukufakwa kobuso ngoketshezi lokuwasha. Ngenxa yomfanekiso, ingaphezulu lefayibha lingacatshangwa njengendawo eqinile ebushelelezi.
B: Ukuqunjelwa uwoyela - I-curling Mechanism
Isinyathelo sesibili esenzweni sokuwasha ukususwa kwamafutha namafutha, ukususwa okungcolile okuwuketshezi kutholakala ngohlobo lokuhlanganisa imali. Ukungcola okuwuketshezi kwasekuqaleni kwakukhona ebusweni ngendlela yefilimu yokusabalalisa yamafutha, futhi ngaphansi komphumela okhethekile wokuwasha owuketshezi endaweni eqinile (ie, ingaphezulu kwefayitela), kwathathelwa indawo ukugeza uketshezi futhi ekugcineni kwaphuma ebusweni ngaphansi kwamandla athile angaphandle.
②shini ◇ yokususwa okuqinile okungcolile
Ukususwa kokungcola okuwuketshezi ikakhulukazi ngokuzikhethela okukhethekile kwesithwali sokungcola ngesisombululo sokuwasha, kuyilapho inqubo yokususa yokungcola okuqinile ihlukile, lapho inqubo yokubhujiswa ihlukile ngokulimala kwesixazululo sokungcola kanye nendawo yayo yenethiwekhi ngesisombululo sokungcola kanye nendawo yayo yenethiwekhi ngesixazululo sokugeza. Ngenxa ye-adsorption of surficants kungcola oqinile kanye nomthwali wayo, ukuxhumana phakathi kokungcola kanye nobuso kuncishisiwe futhi amandla okunamathela esigangeni angcolile ebusweni ancishisiwe, ngakho-ke isisindo sokungcola sisuswa kalula ebusweni bomthwali.
Ngaphezu kwalokho, ama-adsorption of surficaternts, ikakhulukazi ama-ionic sursuctants, ngaphezulu kokungcola okuqinile kanti ophethe awo anamandla okukhulisa amandla angaphezulu komoya oqinile kanye nomthwali wayo, okulungele ukususwa kokungcola okuqinile. Izindawo eziqinile noma eziqinile ze-fibrous zivame ukukhokhiswa kabi kwimidiya yamanzi futhi ngenxa yalokho zingakha izingqimba eziphindwe kabili ze-elektroniki ezindabeni ezingcolile noma ezinhlamvu eziqinile. Ngenxa yokuxoshwa kwamacala amakhulu, ukunamathela kwezinhlayiya ezingcolile emanzini kuya endaweni eqinile kubuthakathaka. Lapho kufakwa i-anionic survictant, ngoba kungakhuphula ngasikhathi sinye okungahle kukhulise amandla angemuhle okungcola okungcolile kanye nendawo eqinile, ukucasulwa okukodwa kuthuthukiswa ngokwengeziwe, amandla okunamathela kuncishiswa kakhulu, futhi ukungcola kulula ukususa.
Ama-surfactants angewona ama-ionic akhishwe nge-adposit eqinile ekhokhwayo ethe xaxa futhi yize azange aguqule kakhulu amandla okusebenzisana, ama-adsorbed an-ionic sursuctants athambekele ekwakheni ukushuba okuthile okusiza ukungcola okuwukungcola.
Endabeni ye-cationic surficcactants, ama-adsorption awo anciphisa noma aqede amandla amabi asebusweni angcolile kanye nendawo yayo yenethiwekhi, enciphisa ukuphazamiseka phakathi kokungcola nobuso futhi ngakho-ke akufanelekela ukususwa okungcolile; Ngaphezu kwalokho, ngemuva kwe-adsorption endaweni eqinile, ama-sursuctants we-catioc ajwayele ukuvula i-hydrophopobic eqinile futhi ngenxa yalokho awafanele ukuwala amanzi futhi ngakho-ke ukugeza.
③ Ukususwa kwenhlabathi ekhethekile
Amaprotheni, isitashi, izithako zomuntu, ujusi wezithelo, ujusi wetiye kanye nokunye ukungcola okunjalo kunzima ukuwususa ngobuningi obujwayelekile futhi kudinga ukwelashwa okukhethekile.
Ama-Protein ama-Protein anjenge-ayisikhilimu, amaqanda, igazi, ubisi kanye nesikhumba excreta athambekele ekuhlanganiseni imicu nokuwohloka futhi athole ukunamathela okunamandla. Amaprotheni alimele angasuswa ngokusebenzisa ama-proteries. I-enzyme protease ibhidliza amaprotheni ekungcoleni ngama-amino acid ancibilika noma ama-oligopeptides.
Amabala wesitashi aqhamuka ikakhulu ekudleni, amanye afana ne-gravy, glue njll inomthelela we-catalytic kwi-hydrolysis yamabala wesitashi, okwenza isitashi sihlukanise ushukela.
I-Liphase Catalyzes ukuwohloka kwama-triglycerides, okunzima ukuwasusa ngezindlela ezijwayelekile, njenge-sebum namafutha adliwayo, futhi kubephula phansi kube yi-lyluble glycerol namafutha acid.
Amanye amabala anemibala avela ezifuyweni zezithelo, ama-withe tices, ama-inks, i-lipstick njll. Kuvame ukuba nzima ukuhlanza kahle ngisho nokugeza okuphindaphindwayo. Lawa ma-stein angasuswa ukusabela kwe-redox nge-oxidizing noma i-ejenti enjenge-bleach, ebhubhisa ukwakheka kwamaqembu akhiqizwa umbala noma umbala-a dengwane awonakalisa ezingxenyeni ezincane ezingenamsebenzi.
(4) Inkambiso yokususa imishini yokuzihlanza eyomile
Okungenhla empeleni kumanzi njengendlela yokugeza. Eqinisweni, ngenxa yezinhlobo ezahlukene zokugqoka nesakhiwo, ezinye izingubo ezisebenzisa ukugeza amanzi akulula noma akulula ukugeza, ukugcwala kwezingubo zemvelo zidonsa amanzi futhi kube lula ukuncibilika, ngakho-ke ukugeza kube lula, ngakho-ke ngemuva kokugeza kuzolungiswa; ngemikhiqizo ye-washing woy ibuye ivele i-shromenon, eminye imikhiqizo yoboya enokugeza amanzi nayo kulula ukuyiphapha, ushintsho lombala; Abanye abalumulululu bezandla bazizwa beba nzima ngemuva kokugeza futhi balahlekelwe yi-luster yabo. Kulezi zingubo zivame ukusebenzisa indlela yokuhlanza eyomile ukuze uhlukanise. Ukuhlanzeka okubizwa ngokuthi owomile ngokuvamile kubhekisele ekuhlanzeni indlela ku-organic solvents, ikakhulukazi kuma-solvents angewona ama-polar.
Ukuhlanza okomile kuyindlela yokugeza emgodini kunokugeza amanzi. Ngoba ukuhlanza okwomile akudingi isenzo esiningi semishini, akubangeli ukulimala, ukugoqa nokukhubazeka kwezingubo, ngenkathi ama-ejenti wokuhlanza owomile, akufani nalawo manzi, akuvamisile ukukhiqizwa. Uma nje ubuchwepheshe buphathwa kahle, izingubo zingahlanzwa ngaphandle kokuhlanekezela, ukuqubuka kombala kanye nempilo yenkonzo eyengeziwe.
Ngokuya kokuhlanza owomile, kunezinhlobo ezintathu ezibanzi zokungcola.
I-①OIL-soluble dirt u-oil-soluble ukungcola kufaka zonke izinhlobo zamafutha namafutha, okuwuketshezi noma okunamafutha futhi kungachithwa kuma-sol sol somile esomile.
I-②Water-Soluble-Soluble Dell-soluble tury-soluble struble incane ngezixazululo zamanzi, kepha hhayi kuma-ejenti wokuhlanza owomile, ama-adsorbed ezingutsheni ezisesimweni esinamanzi, amanzi avele ngemuva kosawoti we-granular, onjengosawoti we-inorganic, isitashi, amaprotheni, njll.
I-③Oil ne-water insoluble udaka uwoyela nokungcola okungafakwanga kwamanzi akwehlile emanzini noma kuncibilike kuma-solvents owomile wokuhlanza, njengekhabhoni emnyama, izimbumbulu zezinsimbi ezimnyama kanye no-njll.
Ngenxa yemvelo ehlukile yezinhlobo ezahlukahlukene zokungcola, kunezindlela ezahlukahlukene zokususa ukungcola kwinqubo yokuhlanza eyomile. Inhlabathi engenakuncibilika kawoyela, njenge-oyela yezilwane kanye nemifino, uwoyela wamaminerali kanye nokuqina, kuncibilika kalula kuma-organic solvents futhi kungasuswa kalula ekuhlanzeni okwomile. I-solubility enhle kakhulu yezinto ezihlanza ezomile zamafutha nokugqeka empeleni zivela ezindongeni ze-van der phakathi kwama-molecule.
Ukususwa kokungcola okuncibilikayo kwamanzi okufana nosawoti we-inorganic, ushukela, amaprotheni kanye nesithukuthuku, inani elifanele lamanzi kumele lingezwe kumenzeli wokuhlanza omile, ngaphandle kokungcola okuncibilikayo kwamanzi kunzima ukuwususa ezingutsheni. Kodwa-ke, amanzi kunzima ukuncibilikisa e-ejenti esomile yokuhlanza, ngakho-ke ukwandisa inani lamanzi, futhi udinga ukwengeza ama-surfactants. Ukuba khona kwamanzi e-ejenti yokuhlanza eyomile kungenza ingaphezulu lokungcola kanye nezingubo hydrate, ukuze kube lula ukusebenzisana namaqembu ama-polar ama-surficaternts, okulungele ama-adsorplants of the surficactants ebusweni. Ngaphezu kwalokho, lapho ama-surffachants enza ama-michelles, ukungcola namanzi okuncibilikayo namanzi kungakhungathekiswa kwiMicellet. Ngaphezu kokukhulisa okuqukethwe kwamanzi kwe-solvent ehlanza eyomile, ama-surfactants angadlala neqhaza ekuvikeleni ukwakhiwa kabusha kokungcola ukungcolisa umphumela wokuqothula.
Ukuba khona kwenani elincane lamanzi kuyadingeka ukususa ukungcola okuncibilikisiwe kwamanzi, kepha amanzi amaningi angadala ukuhlanekezela nokushaya ngezingubo ezithile, ngakho-ke inani lamanzi e-ejenti elomile kufanele lilinganise.
Ukungcola okungahlanganisiwe kwamanzi noma izinhlayiya eziqinile, izinhlayiya eziqinile ezifana nomlotha, udaka, umhlaba kanye nomhlaba omnyama, ngokuvamile kunamathelwe egatsheni ngamandla kagesi noma ngokuhlanganiswa namafutha. Ekuhlanzeni okwomile, ukugeleza kwe-solvent, umthelela ungenza ama-advel adgestatic force adcesp off, futhi i-ejenti yokuhlanza i-oyela, ukungcola kwamafutha okuhlanza, ukungcola okuqinile kube yinhlanganisela yezinhlayiya eziqinile, ukungcola okuqinile, ukuze kufakwe izinhlanzi eziqinile, nokungcola, ukuvikela ukumiswa kwalo kwizingubo.
(5) Izici ezithinta ukugeza isenzo
I-adsorption eqondayo yama-surfactants esibonakalayo kanye nokwehliswa kwento ephezulu (i-interfacial) izici eziphambili ekususweni kodoti oluwuketshezi noma oluqinile. Kodwa-ke, inqubo yokuwasha iyinkimbinkimbi futhi isebenza ngokugeza, noma ngohlobo olufanayo lokuhlanza, lithonywa ezinye izinto eziningi. Lezi zinto zifaka phakathi ukufinyeleleka kwesihlamba, izinga lokushisa, uhlobo lokugcotshwa, uhlobo lwe-fiber kanye nesakhiwo sendwangu.
① Ukuhlushwa Okuqinile
IMicelletes of SurfAntants kwisixazululo idlala indima ebalulekile kwinqubo yokuwasha. Lapho ukugcwala kufinyelela ku-Micelle Cornction (CMC) ebucayi, umphumela wokuwasha ukhulisa kakhulu. Ngakho-ke, ukugcwala kokuhlanza e-solvent kufanele kube ngaphezulu kwenani le-CMC ukuze kube nomphumela omuhle wokuwasha. Kodwa-ke, lapho ukugcwala kwe-surfactant kuphakeme kunenani le-CMC, ukwanda okukhuphukayo ekuhlanzeni umphumela akubonakali futhi akudingekile ukukhulisa ukugcwala okungasebenzi kakhulu.
Lapho ususa uwoyela ngokuthula, umphumela we-solubilization ukhuphuka ngokwengeziwe kokuhlushwa okuqhubekayo, noma ngabe ukugxila kungaphezulu kwe-CMC. Ngalesi sikhathi, kungakuhle ukuthi usebenzise umshini wokuhlanza ngendlela ephakathi nendawo. Isibonelo, uma kunokungcola okuningi ku-cuffs kanye nekhola yengubo, ungqimba lwesiteleka angasetshenziswa ngesikhathi sokugeza ukuze wandise umphumela wokuqina we-oyela.
②Temperature inethonya elibaluleke kakhulu esenzweni sokuqoshwa. Ngokuvamile, ukukhuphula izinga lokushisa kusiza ukususwa kokungcola, kepha kwesinye isikhathi izinga lokushisa lingadala nobubi.
Ukwanda kwezinga lokushisa kusiza ukungcola kokungcola, amafutha aqinile akhungatheka kalula emazingeni okushisa angenhla kwephuzu lawo elincibilikayo futhi imicu yanda ukuvuvukala ngenxa yokuvuvukala kwezinga lokushisa. Kodwa-ke, izindwangu ezihlanganisiwe, ama-microgaps aphakathi kwale micu ancishisiwe njengoba imicu inyuka, okulimaza ukususwa kokungcola.
Izinguquko zokushisa zithinta ne-solubity, inani le-CMC kanye nosayizi we-micelle of surfactants, ngaleyo ndlela ethinta umphumela wokuwasha. I-solubility yama-surfactants enamaketanga amade wekhabhoni iphansi emazingeni okushisa aphansi futhi kwesinye isikhathi i-solubity ingaphansi kakhulu kunenani le-CMC, ngakho-ke izinga lokugeza lishayele kufanele liphakanyiswe ngendlela efanele. Umphumela wokushisa kunani le-CMC kanye nosayizi we-micelle uhlukile ku-Ionic and Non-Ionic Surfactants. Ku-Ionic Surfactants, ukwanda kwezinga lokushisa kuvame ukukhulisa inani le-CMC futhi kunciphise usayizi we-micelle, okusho ukuthi ukugcwala kwe-surving esikhandleni sokuwasha kufanele kukhuphuke. Kwama-surfactants okungewona ama-Ionic, ukwanda kwezinga lokushisa kuholela ekwehleni kwenani le-CMC kanye nokwanda okuphambili kwevolumu ye-Minelle, ngakho-ke kuyacaca ukuthi ukwanda okufanelekile kuzosiza amandla angenayo asebenzayo. Kodwa-ke, izinga lokushisa akufanele lidlule iphuzu laso lefu.
Ngamafuphi, izinga lokushisa elilungile lokuwasha liya ekwakhekeni kwe-detergent kanye nento egezwa. Okunye okokuhlanza kube nomphumela omuhle wokuhlanza emazingeni okushisa asekamelweni, kanti abanye banokuhlanza okuhlukile phakathi kokugeza okubandayo nokushisayo.
③ Foam
Kuyisiko ukudida amandla amagwebu ngokugeza umphumela, ukholelwa ukuthi ama-detergents anamandla amakhulu agwebe anomphumela omuhle wokuwasha. Ucwaningo selukhombisile ukuthi abukho ubudlelwano obuqondile phakathi komphumela wokuwasha kanye nenani lamagwebu. Isibonelo, ukugeza ngezinsimbi eziphansi ze-foaming akunampumelelo ukwedlula ukugeza nge-high fooming detegergents.
Yize igwebu lingahlobene ngokuqondile nokuwasha, kunezikhathi lapho kusiza ukususa ukungcola, ngokwesibonelo, lapho ukugeza izitsha ngesandla. Lapho ama-carpets ekroloki, i-foam nayo ingasusa uthuli nezinye izinhlayiya eziqinile, ukungcola kwekhaphethi ukuze uthole ingxenye enkulu yothuli, ngakho-ke ama-ejenti wokuhlanza amakhaphethi kufanele abe nekhono elithile lokuhlanza.
Amandla we-Foaming nawo abalulekile kuma-shampoos, lapho amagwebu amahle akhiqizwa yi-swampooing noma ukugeza ashiya izizwa ezigcotshwe futhi zintofontofo.
Izinhlobo ④ zemicupho kanye nezindawo ezibonakalayo zezindwangu
Ngaphezu kwesakhiwo samakhemikhali semicu, esithinta ukunamathela nokususwa kokungcola, ukubukeka kwemicu kanye nenhlangano yentambo nendwangu kube nethonya ekususeni okuwukungcola.
Izikali zemicu yoboya kanye nezimbambo ezifuywayo ezigobile zemicu yekotini kungenzeka ziqoqe ukungcola kunemicu ebushelelezi. Isibonelo, i-carbon emnyama emiswe kumafilimu we-cellulose (amafilimu we-viscose) kulula ukuyikhipha, kanti ikhalabholi elimnyama ligcwele izindwangu zekotini kunzima ukuligeza. Esinye isibonelo ukuthi izindwangu ze-fiber ezimfishane ezenziwe nge-polyester zithambekele kakhulu ukuqongelela amabala kawoyela kunezindwangu ezinde, kanye namabala kawoyela ezindwangu ezimfishane futhi kunzima kakhulu ukususa kunamabala e-fiber ende.
Izindwangu ezisontekile ezisontekile nezindwangu eziqinile, ngenxa yegebe elincane eliphakathi kwemicu, kepha okufanayo kungavimba nokugeza uketshezi ngaphandle kokungcola kwangaphakathi, kodwa izindwangu eziqinile ziqala ukumelana nokungcola okuhle.
⑤ Ubulukhuni bamanzi
Ukuhlushwa kwe-CA2 +, i-MG2 + namanye ama-ion wensimbi emanzini kunomthelela omkhulu emvuzweni wokuwasha, ikakhulukazi lapho ama-anionic extractants ahlangana khona, ikakhulukazi lapho ama-anionic e-eon ahlangana ne-calcium ne-magnosium elungisa ama-calcium kanye nosawoti we-magnosium ancibilikayo futhi ancishiswe ukungcola kwalo. Ngamanzi anzima, noma ngabe ukugcwala kwe-surfactant kuphezulu, ukungcola kusekubi kakhulu kunasekuhlotshiswe. Ukuze i-surfactant yokuba nomphumela omuhle kakhulu wokuwasha, ukugcwala kwe-CA2 + i-ion emanzini kufanele kwehliswe ku-1 x 10-6 mol / l (caco3 kuye ku-0,1 mg / l) noma ngaphansi. Lokhu kudinga ukungezwa kwabathambisi abahlukahlukene kuya esihlalweni.
Isikhathi sePosi: Feb-25-2022



